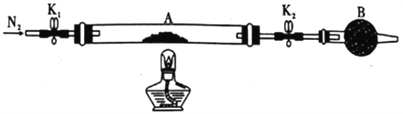
��Ŀ����
����Ŀ��ij��ѧ��ȤС��������ͼװ��̽������Zn��һ����Ũ���ᷴӦ����������ɷ֣�ˮ�������⣩��

��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ_______________��
��2����������������������˳��Ϊ��a������_____����______������______����______������_____����_____������______����______����β���������������ӿ���ĸ��ţ���
��3��ʢ��Ũ�������������Ϊ________����������ʹ��ǰӦϴ����______________��
��4���Լ�XΪ_________________��
��5��װ��E������Ϊ____________________��
��6��װ��C������Ϊ________��֤����װ���з�Ӧ�����Ե�ʵ�����������Ϊ_____________��
��7��ʵ��ʱ��ӦʹAװ���������ȷ�Ӧһ��ʱ�䣬�ٵ�ȼB���ƾ��ƣ�ԭ��Ϊ___________��
���𰸡� Zn+2H2SO4��Ũ��=ZnSO4+SO2��+2H2O��Zn+2H2SO4=ZnSO4+H2�� d e h i b c f g ��Һ©�� �����Ƿ�©Һ CuSO4��������ͭ������ˮ����ͭ�� ����SO2������H2�����ȥSO2��ˮ������ ����SO2 ������ɫ���Ʒ����Һ����Һ�ָ���ɫ �ų�Cװ���еĿ�������ֹH2�������ϼ��ȷ���Σ��
��������������Ҫ������ڡ�̽������Zn��һ����Ũ���ᷴӦ����������ɷ֡�̽��ʵ������ۡ�
��1��A�з�����Ӧ�Ļ�ѧ����ʽΪZn+2H2SO4��Ũ��=ZnSO4+SO2��+2H2O��Zn+2H2SO4=ZnSO4+H2����
��2������������A������������ͨ��C�������������ͨ��E��ȥ��������ˮ��������ͨ��B��D��������������������˳��Ϊ(a������d����e������h����i������b����c������f����g����β��������
��3��ʢ��Ũ�������������Ϊ��Һ©������������ʹ��ǰӦϴ������©��
��4���Լ�XΪCuSO4��
��5��װ��E������Ϊ����SO2������H2�����ȥSO2��ˮ��������
��6��װ��C������Ϊ����SO2��֤����װ���з�Ӧ�����Ե�ʵ�����������Ϊ������ɫ���Ʒ����Һ����Һ�ָ���ɫ��
��7��ʵ��ʱ��ӦʹAװ���������ȷ�Ӧһ��ʱ�䣬�ٵ�ȼB���ƾ��ƣ�ԭ��Ϊ�ų�Cװ���еĿ�������ֹH2�������ϼ��ȷ���Σ����

 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�