��Ŀ����
��X��Y��Z���ֶ�����Ԫ�أ�X����̬�⻯�ﻯѧʽΪH2X�����⻯���ʽ����X����������ʽ��֮��Ϊ17��40��Xԭ�Ӻ�������������������ȣ�Y��X�����γ����ӻ�����Y2X��Y�������ӵ��Ӳ�ṹ��Ne��ͬ��Z��Xͬ���ڣ�����̬������˫ԭ�ӷ��ӣ���ԭ�ӹ���1�Ե��ӡ��Իش�(1)д����Ԫ�ط��ţ�X________��Y________��Z________��
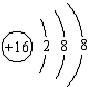
(2)X���ӵĽṹ��ͼΪ________��X��Y�γɵ����ӻ�����ĵ���ʽΪ________��Z�����γɵĻ�����ĵ���ʽΪ________________��
(3)Y�����ڿ�����ȼ�յĻ�ѧ����ʽΪ____________________________________����������ˮ��Ӧ�Ļ�ѧ����ʽΪ_________________________________________________��
�𰸣�
������
��ʾ��
������
| (1)S�� Na��
Cl
(2) (3)2Na+O2
|
��ʾ��
| X�ĸ���Ϊ�������������Ϊ+6,������Ԫ�أ��ָ�����ʽ���ȣ��������ԭ������ȷ����Ԫ��ΪS��Y����һ�۵ļ����Ԫ�أ������Ӻ�����ʮ�����ӣ��ʿ���ȷ������Na��Z��������Ԫ�أ���Cl��
|

��ϰ��ϵ�д�
�����Ŀ
 ��
��