��Ŀ����
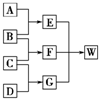
A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʡ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ����������������ͼ��ת����ϵ(��Ӧ������)��
(1)��֪EΪֱ���εķǼ��Է��ӣ���E�ĽṹʽΪ______________��D���ʵĵ���ʽΪ_______________��G����Ԫ�ص�ԭ�Ӹ�����Ϊ1��3����G���ӵĿռ乹��Ϊ______________��
(2)C+D![]() G�Ļ�ѧ����ʽΪ__________________________________________��
G�Ļ�ѧ����ʽΪ__________________________________________��
(3)��֪������Ԫ��X��B�е�Ԫ��λ��ͬһ���壬��X������������Ӧˮ�����Ũ��Һ��A��Ӧ�Ļ�ѧ����ʽΪ____________________________________��
(4)
(1)O=C=O ![]() ������
������
(2)N2+3H2![]() 2NH3
2NH3
(3)2H2SO4(Ũ)+C![]() CO2��+2H2O+2SO2��
CO2��+2H2O+2SO2��
(4)CO2+NH3+NaCl+H2O![]() NaHCO3��+NH4Cl
NaHCO3��+NH4Cl
����������ΪԪ�ص��ʼ�������Ŀ�ͼ�ƶ���.��AΪ���壬�����嵥��B��Ӧ����EΪֱ���ηǼ��Է��ӣ����ƶ�A��C��B��O2��C��H2��D��N2����EΪCO2��FΪ H2O��GΪNH3�����ӻ�����WΪNH4HCO3.

��ϰ��ϵ�д�
 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
�����Ŀ
 A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
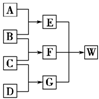 A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���