��Ŀ����
��7�֣�R��Q��X��Y��Z��Ϊ������Ԫ�أ���ԭ����������������֪QԪ������Ȼ������ɵĻ�����������ࣻYԭ����������������������֮��Ϊ 3:4 ��R��Y��Z��Y�������ԭ�Ӹ�����Ϊ1:1��2:1�����ֻ�����ݴ˻ش�
��1����R��X��Y����Ԫ�ؿ���ɶ��ֻ����д�����ֳ����IJ�ͬ���͵Ļ�����Ļ�ѧʽ
___________��______________��
��2����Q��X��ɻ�����(QX)2�У�Q��Xԭ�Ӿ����������8���ӽṹ����ĵ���ʽ��(QX)2______��
��3����֪R��Q����ɻ�����ף�R��Y����ɻ������ң�����һ��������10�����ӣ���һ���ҷ�����18�����ӡ�
�ټĽṹʽ��_____________���ҷ����й��ۼ�����_____________��
�ڼ���һ����Ҫ����Դ��ͨ��״���£�1mol ���ڿ�������ȫȼ�����ɶ�����̼��ˮ���ų�890kJ���ȣ�����ʱȼ��40g�ļף�������������________kJ��
�������������£���FeI2�ĵ�����Һ�м����ң���Һ��������1 mol Fe2+��������Fe3+ʱ����Ӧ�е���ת��Ϊ4 mol���÷�Ӧ�����ӷ���ʽ��_____________________________________________��
��1����R��X��Y����Ԫ�ؿ���ɶ��ֻ����д�����ֳ����IJ�ͬ���͵Ļ�����Ļ�ѧʽ
___________��______________��
��2����Q��X��ɻ�����(QX)2�У�Q��Xԭ�Ӿ����������8���ӽṹ����ĵ���ʽ��(QX)2______��
��3����֪R��Q����ɻ�����ף�R��Y����ɻ������ң�����һ��������10�����ӣ���һ���ҷ�����18�����ӡ�
�ټĽṹʽ��_____________���ҷ����й��ۼ�����_____________��
�ڼ���һ����Ҫ����Դ��ͨ��״���£�1mol ���ڿ�������ȫȼ�����ɶ�����̼��ˮ���ų�890kJ���ȣ�����ʱȼ��40g�ļף�������������________kJ��
�������������£���FeI2�ĵ�����Һ�м����ң���Һ��������1 mol Fe2+��������Fe3+ʱ����Ӧ�е���ת��Ϊ4 mol���÷�Ӧ�����ӷ���ʽ��_____________________________________________��

��

��ϰ��ϵ�д�
�����Ŀ



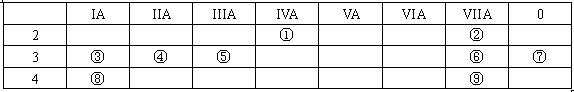 ��1�����Ԫ�ط�����__________
��1�����Ԫ�ط�����__________