��Ŀ����
 ��2010?�ϲ�һģ���ں��º��ݵ��ܱ�������ͨ��1molN2��XmolH2���������·�Ӧ��N2��g��+3H2��g��
��2010?�ϲ�һģ���ں��º��ݵ��ܱ�������ͨ��1molN2��XmolH2���������·�Ӧ��N2��g��+3H2��g��| ���� | ���¡���ѹ |
��֪���ٷ�Ӧ���������仯����ͼ���ڴﵽƽ��ʱ����÷�Ӧ�ų�������Ϊ18.4KJ������������ʵ���Ϊ3.6moL��������ѹǿ��С10%��
��ش��������⣺
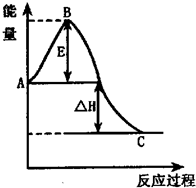
��1��ͼ��A���ʾ
��Ӧ����е�������
��Ӧ����е�������
��C���ʾ��������е�������
��������������
����2���÷�Ӧͨ��ʹ����Ϊ����ĸ��ϴ�����ʹ�øô���ʱ��B��ı仯��
����
����
������ߡ����͡�����3��XֵΪ
3
3
����4���÷�Ӧ���Ȼ�ѧ����Ϊ
N2��g��+3H2��g��?2NH3��g����H=-92kJ/moL
N2��g��+3H2��g��?2NH3��g����H=-92kJ/moL
����5������ʼʱ�����N2��H2��NH3���ʵ����ֱ�Ϊa��b��c��ƽ��ʱ����ֵ���������ƽ�����ȣ���ʼ��ά�ַ�Ӧ����������У�����ʼʱc��ȡֵ��ΧΪ
O��C��0.4
O��C��0.4
����������1���������仯ͼ��֪A��C��ĺ��壻
��2���Ӵ����Ի�ܵ�Ӱ�쿼�ǣ�
��3������ѹǿ�����ʵ����Ĺ�ϵ����x��
��4���ɣ�3����÷ų�18.4KJ�������ʵ����ı仯��������ð���ѧ��������Ӧ����ЧӦ���Ӷ�д���Ȼ�ѧ����ʽ��
��5�����ݣ�4���ļ��㼰ƽ�������ƶ������ǣ�
��2���Ӵ����Ի�ܵ�Ӱ�쿼�ǣ�
��3������ѹǿ�����ʵ����Ĺ�ϵ����x��
��4���ɣ�3����÷ų�18.4KJ�������ʵ����ı仯��������ð���ѧ��������Ӧ����ЧӦ���Ӷ�д���Ȼ�ѧ����ʽ��
��5�����ݣ�4���ļ��㼰ƽ�������ƶ������ǣ�
����⣺��1���������仯ͼ��֪��A�������Ӧ����е���������C�������������е����������ʴ�Ϊ����Ӧ����е������� ��������е���������
��2��ʹ�ô����������˷�Ӧ�����ܣ�����Ӱٷ������ӣ���Ӧ���ʼӿ죬�ʴ�Ϊ�����ͣ�
��3������ѹǿ֮�ȵ������ʵ���֮�ȵã�
��100%=10%�����X=3���ʴ�Ϊ��3��
��4�����ݷ���ʽ�����H
���ʵ����仯Ϊ��1+3-3.6=0.4���ų�18.4KJ���������ʵ����仯Ϊ��1+3-2=2ʱ����Ӧ�������仯ΪX
N2��g��+3H2��g��?2NH3��g����n��Q
1 3 2 2 X
0.4 0.4 18.4
X=
=92������H=-92kJ/moL���ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92kJ/moL��
��5�����ݣ�4����֪��ƽ��ʱNH3�����ʵ�������0.4mol��Ҫ����ƽ�������ƶ�����NH3�����ʵ���ӦС��0.4mol��
�ʴ�Ϊ��O��C��0.4��
��2��ʹ�ô����������˷�Ӧ�����ܣ�����Ӱٷ������ӣ���Ӧ���ʼӿ죬�ʴ�Ϊ�����ͣ�
��3������ѹǿ֮�ȵ������ʵ���֮�ȵã�
| 1+X-3.6 |
| 1+X |
��4�����ݷ���ʽ�����H
���ʵ����仯Ϊ��1+3-3.6=0.4���ų�18.4KJ���������ʵ����仯Ϊ��1+3-2=2ʱ����Ӧ�������仯ΪX
N2��g��+3H2��g��?2NH3��g����n��Q
1 3 2 2 X
0.4 0.4 18.4
X=
| 2��18.4 |
| 0.4 |
��5�����ݣ�4����֪��ƽ��ʱNH3�����ʵ�������0.4mol��Ҫ����ƽ�������ƶ�����NH3�����ʵ���ӦС��0.4mol��
�ʴ�Ϊ��O��C��0.4��
�����������ۺϿ����˷�Ӧ���뻯ѧƽ��ļ����⣮�������ò������ɿ�����⣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2010?�ϲ�һģ������HCl��Ba��OH��2��Na2CO3��NaHCO3�������ʵij�����Һ������֮������ͼ��ʾ�ķ�Ӧ��ϵ��ͼ��ÿ�������˵����ʾ������Ӧ�������ƶ���ȷ���ǣ�������
��2010?�ϲ�һģ������HCl��Ba��OH��2��Na2CO3��NaHCO3�������ʵij�����Һ������֮������ͼ��ʾ�ķ�Ӧ��ϵ��ͼ��ÿ�������˵����ʾ������Ӧ�������ƶ���ȷ���ǣ�������