��Ŀ����
��һ��ɫ��Һ�����ܺ���K+��Al3+��Mg2+��NH4+��Cl-��SO42-��HCO3-��MnO4-�е�һ�ֻ��֣�Ϊȷ����ɷ֣���������ʵ�飺
��ȡ������Һ����ε���NaOH��Һ��������ֻ�۲쵽��ɫ������������ֲ����ܽ⣬��������������
����ȡ������Һ������HNO3�ữ��Ba��NO3��2��Һ������������
�����ƶ���ȷ����
- A.�϶���Al3+��Mg2+��Cl-��������K+
- B.�϶���Al3+��Mg2+��Cl-��������NH4+
- C.�϶���Al3+��Mg2+��HCO3-���϶�û��MnO4-
- D.�϶���K+��Al3+��MnO4-��������HCO3-��
A
����������Һ����ɫ��Һ�ж�һ������MnO4-��ȡ������Һ����ε���NaOH��Һ��������ֻ�۲쵽��ɫ������������ֲ����ܽ⣬��������������˵��һ������Al3+��Mg2+���������ӹ����ж�һ��������HCO3-����Ϊþ���Ӻ��������������ɳ��������ڼ���ɵ������������ڼ笠����Ӻ����������ӽ�Ͽ����������就��������һ������NH4+��
����ȡ������Һ������HNO3�ữ��Ba��NO3��2��Һ�������������ж�һ����SO42-��������Һ�ĵ����ԣ���Һ�е�������һ����Cl-��
�����Һ����ɫ��Һ�ж�һ������MnO4-����ȡ������Һ����ε���NaOH��Һ��������ֻ�۲쵽��ɫ������������ֲ����ܽ⣬��������������˵��һ������Al3+��Mg2+���������ӹ����ж�һ��������HCO3-����Ϊþ���Ӻ��������������ɳ��������ڼ���ɵ������������ڼ笠����Ӻ����������ӽ�Ͽ����������就��������һ������NH4+��
����ȡ������Һ������HNO3�ữ��Ba��NO3��2��Һ�������������ж�һ����SO42-��������Һ�ĵ����ԣ���Һ�е�������һ����Cl-��
������������Һ��һ������Al3+��Mg2+��Cl-��һ����NH4+��SO42-��HCO3-��MnO4-��������K+
��ѡA��
���������⿼�������Ӽ���ģ���Ӧ������жϣ����ӹ����Ӧ�ã���Ҫ��ͬ�����ӵ�����Ӧ�ã���Ŀ�Ѷ��еȣ�
����������Һ����ɫ��Һ�ж�һ������MnO4-��ȡ������Һ����ε���NaOH��Һ��������ֻ�۲쵽��ɫ������������ֲ����ܽ⣬��������������˵��һ������Al3+��Mg2+���������ӹ����ж�һ��������HCO3-����Ϊþ���Ӻ��������������ɳ��������ڼ���ɵ������������ڼ笠����Ӻ����������ӽ�Ͽ����������就��������һ������NH4+��
����ȡ������Һ������HNO3�ữ��Ba��NO3��2��Һ�������������ж�һ����SO42-��������Һ�ĵ����ԣ���Һ�е�������һ����Cl-��
�����Һ����ɫ��Һ�ж�һ������MnO4-����ȡ������Һ����ε���NaOH��Һ��������ֻ�۲쵽��ɫ������������ֲ����ܽ⣬��������������˵��һ������Al3+��Mg2+���������ӹ����ж�һ��������HCO3-����Ϊþ���Ӻ��������������ɳ��������ڼ���ɵ������������ڼ笠����Ӻ����������ӽ�Ͽ����������就��������һ������NH4+��
����ȡ������Һ������HNO3�ữ��Ba��NO3��2��Һ�������������ж�һ����SO42-��������Һ�ĵ����ԣ���Һ�е�������һ����Cl-��
������������Һ��һ������Al3+��Mg2+��Cl-��һ����NH4+��SO42-��HCO3-��MnO4-��������K+
��ѡA��
���������⿼�������Ӽ���ģ���Ӧ������жϣ����ӹ����Ӧ�ã���Ҫ��ͬ�����ӵ�����Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ




 ��1����ԭ��Һ��һ�������ڵ������� ��
��1����ԭ��Һ��һ�������ڵ������� ��
 �������е�һ�ֻ��֡��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ�����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ����ͼ����ʾ��
�������е�һ�ֻ��֡��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ�����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ����ͼ����ʾ��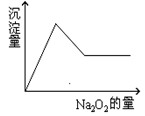

 ��1����ԭ��Һ��һ�������ڵ�������
��
��1����ԭ��Һ��һ�������ڵ�������
��