��Ŀ����
��9�֣���25��ʱ������0.1mol��L-1�İ�ˮ����ش��������⣺
��1������ˮ�м�����������粒��壬��ʱ��Һ���� �����������С�����䡱����
��2������ˮ�м���pH=1�����ᣬ�Ұ�ˮ������������Ϊ1��1����ʱ��Һ��pH 7������ڡ�����С�ڡ����ڡ����������ӷ���ʽ��ʾ��ԭ��
����ʱ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��3������ˮ�м���0.05mol��L-1ϡ��������Һ���ó����ԣ����ð�ˮ�����V1��ϡ��������V2�Ĺ�ϵΪV1 V2������ڡ�����С�ڡ����ڡ�����д����Һ�и�����Ũ��֮������ĵ���غ����ʽ ��
(1)����1�֣�
��2��С�ڣ�1�֣� NH4+ +H2ONH3��H2O+H+��2�֣�
c(NH4+)��c(SO42- )��c(H+)��c(OH-)(2�֣�
��3�����ڣ�1�֣� c(NH4+)+c(H+)=2c(SO42- )+c(OH-)��2�֣�
����:��1����ˮ�д��ڵ���ƽ�⣺NH3��H2ONH4+ +OH-������ˮ�м�����������粒��壬�������c(NH4+)�����Ƶ��룬��ƽ���c(NH4+)�Ա�ԭƽ�����Һ��
������
��2��pH=1�����ᣬ��Ũ����0.05mol��L-1��ͨ�������֪�������϶���ǡ�÷�Ӧ����������泥������ˮ�������ԡ�����ʽΪNH4+ +H2ONH3��H2O+H+��
��3���ɣ�2����֪�������Ϻ���Һ�����ԣ�����Ҫ����Һ�����ԣ���ˮ�������������ˮ�������������������

 NH3?H2O+H+
NH3?H2O+H+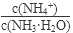 ��
�����������С�����䡱����
��
�����������С�����䡱����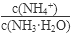 ��
�����������С�����䡱����
��
�����������С�����䡱����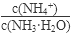 ��
�����������С�����䡱����
��
�����������С�����䡱����