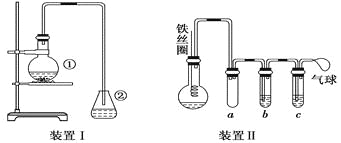
��Ŀ����
����Ŀ�����Ǻϳɵ⻯��Ļ���ԭ�ϡ�������������±ˮ�������������£�

����˵����ȷ����( )
A.��������ʱ����ͨ�����Cl2��֤I�����������
B.�������������ڴ������н��У�Ӧ������±ˮ�������룬�ȿ�������������
C.�������������У����������������ˮ��Һ��ֽӴ�������������
D.�������������У���SO2������ҺҲ������NaOH��Һ���棬���ɵ�I����IO3�����ữ�����ɵôֵ⡣ʹ��NaOH���պ���Һ��I����IO3�������ʵ���֮��Ϊ1��5
���𰸡�C
��������
A��Cl2���ܹ���������Cl2�ὫI2����ΪHIO3��A����
B����������������Ӧ������±ˮ���������룬�ȿ��������״��룬B����
C���������������У����������������ˮ��Һ��Ӧ��I2+SO2+2H2O=2HI+H2SO4��C��ȷ��
D���������������У�NaOH��Һ���溬��SO2������Һ��������Ӧ��3I2+NaOH=5NaI+NaIO3+3H2O������NaOH���պ���Һ��I����IO3�������ʵ���֮��Ϊ5��1��D����
��ѡC��

����Ŀ������β���е�NO�ǻ�����ѧ�о����ȵ���⡣
I��NO��������
��֪��2NO(g)+O2(g) ![]() 2NO2(g)��H=-110kJ��mol-1
2NO2(g)��H=-110kJ��mol-1
25��ʱ����NO��O2�����ʵ���֮��Ϊ2:1������ݷ�Ӧ�����У��ò�ѹ���о��䷴Ӧ�Ľ����������ϵ����ѹǿp��ʱ��t�ı仯���±���ʾ(����NO2��N2O4��ת��)
t/min | 0 | 80 | 160 |
|
p/kPa | 75.0 | 63.0 | 55.0 | 55.0 |
��1��0~80min��v(O2)=_____kPa/min�����ŷ�Ӧ���У���Ӧ������С��ԭ����______________��
��ƽ���ѹ����ƽ��Ũ�����õ���ƽ�ⳣ����K(p)��ʾ��25��ʱ��K(p)��ֵΪ_______(����3λ��Ч����)��
��2���������ϣ������ܷ�Ӧ2NOg)+O2(g) ![]() 2NO2(g)��������������
2NO2(g)��������������
��һ��2NO(g) ![]() N2O2(g) ���ٷ�Ӧ
N2O2(g) ���ٷ�Ӧ
�ڶ���N2O2(g)+O2(g) ![]() 2NO2(g) ����Ӧ
2NO2(g) ����Ӧ
�ܷ�Ӧ������Ҫ�ɵ�_____�������������÷��Ӳ������ʵ����ٷ�Ӧ�����е�N2O2���ܷ�Ӧ��ƽ�ⳣ��K(p)��___(����������������С������������)������߷�Ӧ�¶���35��������ϵѹǿP(35��)______P(25��)(������������������������С����)��
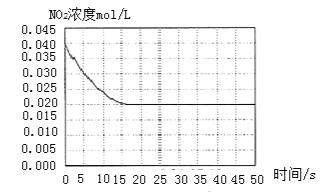
II. ij�¶���һ�ܱ������г���һ������NO2�����NO2Ũ����ʱ��仯����������ͼ��ʾ��
��1����Ӧ��ϵ��ƽ���ѹǿΪP1���������¶ȣ��ٴδ�ƽ���������ƽ����Է�������______![]() ����������������С������������
����������������������������![]() ��
��
��2�����ں��º��������£���ƽ����ϵ�г���һ����O2���ٴδ�ƽ����ѹǿΪP2��c(O2)=0.09mol/L����P2��P1=______
��3�����¶��·�Ӧ2NO(g)+O2(g) ![]() 2NO2(g)�Ļ�ѧƽ�ⳣ��KΪ______��
2NO2(g)�Ļ�ѧƽ�ⳣ��KΪ______��