��Ŀ����
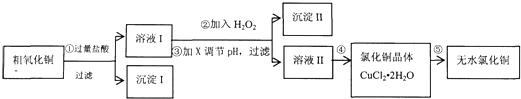
��16�֣�ijͬѧ���ô�����ͭ��������FeO��������������ʣ���ȡ��ˮ�Ȼ�ͭ��������ͼ��
(1)�����������ͭ�����ᷴӦ���������ʽ��___________________��
(2)������м���H2O2��Ŀ��____________________________________��
����II�Ļ�ѧʽΪ_______________________��
(3)��֪��
| | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
| Fe2+ | 7 | 9 |
a.NaOH b.CuO c.Cu(OH)2 d.

(4)����ܵIJ�����___________�����ˡ�ϴ�ӡ����
Ϊ�õ���ˮCuCl2����������ڸ����HCl�����м���
 ��ԭ����__________________________________________________________��
��ԭ����__________________________________________________________��
(1)CuO��2H��=Cu2����H2O��2�֣���
(2)��Fe2+������Fe3+��2�֣��� Fe(OH)3��2�֣���
(3)3.2��4.7֮�䣨2�֣��� bc��4�֣�ѡһ���Ե�2�֣�һ��һ�����÷֣���
(4)����Ũ������ȴ�ᾧ��2�֣�����ֹCuCl2����ˮ�⣨2�֣�
����

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д���16�֣�ijͬѧ���ô�����ͭ������������������������������ʣ���ȡ��ˮ�Ȼ�ͭ��������ͼ��ʾ��
��1����������漰�����ӷ���ʽ�У� ��
��2��������м���H2O2��Ŀ���ǣ� ��Ӧ�����ӷ���ʽΪ ��
��3����֪��
| �� | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3�� | 1.9 | 3.2 |
| Cu2�� | 4.7 | 6.7 |
| Fe2�� | 7 | 9 |
������п������ڵ�����ҺpH���Լ�X�� ��
a��Cu2(OH)2CO3 b��CuO c�� Cu(OH)2 d��NH3?H2O
��4������ܽ��еIJ����� �����ˡ�ϴ�ӡ���� �ڲ������Ҫ�õ���ˮCuCl2����Ҫ�� ����CuCl2��2H2O��
��16�֣�ijͬѧ���ô�����ͭ������������������������������ʣ���ȡ��ˮ�Ȼ�ͭ��������ͼ��ʾ��

��1����������漰�����ӷ���ʽ�У� ��
��2��������м���H2O2��Ŀ���ǣ� ��Ӧ�����ӷ���ʽΪ ��
��3����֪��
|
�� |
�������↑ʼ����ʱ��pH |
�������������ȫʱ��pH |
|
Fe3�� |
1.9 |
3.2 |
|
Cu2�� |
4.7 |
6.7 |
|
Fe2�� |
7 |
9 |
������е���pH����ѷ�ΧΪ ��
������п������ڵ�����ҺpH���Լ�X�� ��
a��Cu2(OH)2CO3 b��CuO c�� Cu(OH)2 d��NH3•H2O
��4������ܽ��еIJ����� �����ˡ�ϴ�ӡ���� �ڲ������Ҫ�õ���ˮCuCl2����Ҫ�� ����CuCl2��2H2O��
��16�֣�ijͬѧ���ô�����ͭ��������FeO��������������ʣ���ȡ��ˮ�Ȼ�ͭ��������ͼ��

(1)�����������ͭ�����ᷴӦ���������ʽ��___________________��
(2)������м���H2O2��Ŀ��____________________________________��
����II�Ļ�ѧʽΪ_______________________��
(3)��֪��
|
|
�������↑ʼ����ʱ��pH |
�������������ȫʱ��pH |
|
Fe3+ |
1.9 |
3.2 |
|
Cu2+ |
4.7 |
6.7 |
|
Fe2+ |
7 |
9 |
������е���pH����ѷ�ΧΪ____��������ҺpH���Լ�X������______________:
a.NaOH
b.CuO c.Cu(OH)2
d.
(4)����ܵIJ�����___________�����ˡ�ϴ�ӡ����
Ϊ�õ���ˮCuCl2����������ڸ����HCl�����м��� ��ԭ����__________________________________________________________��
��ԭ����__________________________________________________________��