��Ŀ����
����Ŀ����ϩ��һ����Ҫ�Ļ�������ԭ�ϣ�����Ϊԭ�ϻ����Ժϳɺܶ�Ļ�����Ʒ����������ת����

��1������ϩ�Ľṹ��ʽΪ ��
��2��д�����·�Ӧ�Ļ�ѧ����ʽ�ͷ�Ӧ���ͣ�
�� ����Ӧ������
�� ����Ӧ������
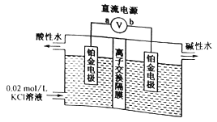
��3��ʵ��������ͼװ���Ʊ������������Թ������Ƭ�������� ������a�������ǵ����� ���Թ�b�ڱ���Na2CO3��Һ������һ�����dz�ȥ���������л��е�������Ҵ�����һ������ ��

���𰸡���1��![]() ��
��
��2��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��������Ӧ
2CH3CHO+2H2O��������Ӧ
CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O�� ȡ����Ӧ/������Ӧ
CH3COOCH2CH3+H2O�� ȡ����Ӧ/������Ӧ
��3����ֹҺ�屩�У��������������������������ܽ�ȣ�
��������
����������������ת����ϵ�ƶϣ�BΪCH3CH2OH��CΪCH3CHO��DΪCH3COOH��
��1����ϩ�����ӳɾۺϷ�Ӧ���ɾ���ϩ������ϩ�Ľṹ��ʽΪ![]() ��
��
��2����Ӧ��Ϊ�Ҵ���������ͭ�����������ȵ������·���������Ӧ������ȩ��ˮ����ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ӧ��Ϊ�Ҵ���������Ũ���������������ȵ������·���������Ӧ����ȡ����Ӧ����������������ˮ����ѧ����ʽΪCH3COOH+CH3CH2OH
2CH3CHO+2H2O����Ӧ��Ϊ�Ҵ���������Ũ���������������ȵ������·���������Ӧ����ȡ����Ӧ����������������ˮ����ѧ����ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��3��ʵ��������ͼװ���Ʊ������������Թ������Ƭ����������ֹҺ�屩��������a�������ǵ����������������Թ�b�ڱ���Na2CO3��Һ������һ�����dz�ȥ���������л��е�������Ҵ�����һ���������������������ܽ����

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�����Ŀ���������±������ϣ��ش��������⣺
�����������������Ŀ���ʴ����Խ��Խ�ܵ����ǵ��i��������Ҫ����������������ͭ��̼��Ԫ������ɵĺϽ�
�����ࣨGe���ǵ��͵İ뵼��Ԫ�أ��ڵ��ӡ����ϵ�����Ӧ�ù㷺��
�����黯�أ�GaAs���������İ뵼����ϣ������������ͼ�������̫���ܵ�صIJ��ϵȡ�
����K2Cr2O7�����ڼ��˾���Ƿ�ƺ��ʻ��
Cr2O72-(��ɫ)+CH3CH2OH![]() Cr3+(��ɫ)+CH3COOH (δ��ƽ��
Cr3+(��ɫ)+CH3COOH (δ��ƽ��
��1����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_________________��
��2��CH3COOH����������Ԫ�صĵ縺���ɴ�С��˳��Ϊ___________��̼ԭ�ӵĹ���ӻ�����Ϊ_________��������������������Ŀ֮��Ϊ______________________��
��3��AsCl3���ӵ����幹��Ϊ_____________����ԭ������_________��δ�ɶԵ��ӡ�
��4�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ����[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ______���ṩ�µ��ӶԵ�ԭ����_____��
��5���Ƚ�������±������۵�ͷе㣬������仯���ɼ�ԭ��____________________��
GeCl4 | GeBr4 | GeI4 | |
�۵�/�� | 49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
��6��ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��

�پ�����ͭԭ������ԭ�ӵ�������Ϊ_____��
�����Ͻ���ܶ�Ϊdg/cm3����������a=________nm