��Ŀ����
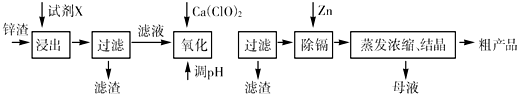
��п�������Ĺ�ҵ����--п�������˺�Zn�⣬������Fe��Al��Cd��SiO2�����ʣ�������п����ȡ������ZnSO4?7H2O�ͽ�������һ������ij��ԣ����������£�

��֪��Fe3+��Al3+��Zn2+��Cd2+��Fe2+������������ȫ����ʱ��pH�ֱ�Ϊ��3.2��4.7��6.5��9.4��9.7
�Իش��������⣺
��1������ʱ�õ����Լ�XΪ______
��2��д������Ca��ClO��2��Ӧ�����ӷ���ʽ______
��3������pH���̿���ѡ��______���ZnO����NaOH�����������̵�pH����һ�����5����Ŀ����______
��4��д�����˺���������п�۷�Ӧ�����ӷ���ʽ______
��5��������Ũ������ʱ��Ҫ��ȡʵ���ʩ______��

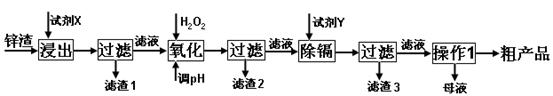
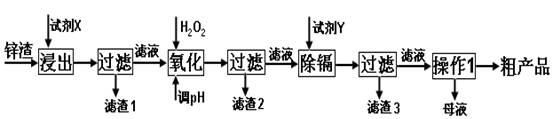
��֪��Fe3+��Al3+��Zn2+��Cd2+��Fe2+������������ȫ����ʱ��pH�ֱ�Ϊ��3.2��4.7��6.5��9.4��9.7
�Իش��������⣺
��1������ʱ�õ����Լ�XΪ______
��2��д������Ca��ClO��2��Ӧ�����ӷ���ʽ______
��3������pH���̿���ѡ��______���ZnO����NaOH�����������̵�pH����һ�����5����Ŀ����______
��4��д�����˺���������п�۷�Ӧ�����ӷ���ʽ______
��5��������Ũ������ʱ��Ҫ��ȡʵ���ʩ______��
��1����������ȡ����п����ģ�����Ӧ����ϡ���ᣬ�ʴ�Ϊ��H2SO4��Һ��
��2��Ca��ClO��2�����������ӣ�����˫��ˮ�Ļ�ԭ������ˮ�����Է���ʽΪ2H++ClO-+2Fe2+=Cl-+2Fe3++H2O��
�ʴ�Ϊ��2H++ClO-+2Fe2+=Cl-+2Fe3++H2O��
��3�����������µ����ʣ�����ѡ������п�������NaOH�����������ᵼ��Zn2+����������
�ʴ�Ϊ��ZnO����ȥFe3+��Al3+����ֹZn2+����������
��4��п�Ľ�����Ա���ǿ��������Ҫ�û����ӣ��ֲ����������ʣ�ѡ��п����Ӧ�����ӷ���ʽΪZn+Cd2+=Zn2++Cd��
�ʴ�Ϊ��Zn+Cd2+=Zn2++Cd��
��5��Ҫ����Һ�еõ�����п���壬Ӧ����ͨ������Ũ���������ᾧ��Ȼ����ˣ�ϴ�Ӽ��ɣ�Ϊ��ֹ����пˮ����������ʣ�Ӧ����һ������ȣ��ʴ�Ϊ������һ������ȣ�
��2��Ca��ClO��2�����������ӣ�����˫��ˮ�Ļ�ԭ������ˮ�����Է���ʽΪ2H++ClO-+2Fe2+=Cl-+2Fe3++H2O��
�ʴ�Ϊ��2H++ClO-+2Fe2+=Cl-+2Fe3++H2O��
��3�����������µ����ʣ�����ѡ������п�������NaOH�����������ᵼ��Zn2+����������
�ʴ�Ϊ��ZnO����ȥFe3+��Al3+����ֹZn2+����������
��4��п�Ľ�����Ա���ǿ��������Ҫ�û����ӣ��ֲ����������ʣ�ѡ��п����Ӧ�����ӷ���ʽΪZn+Cd2+=Zn2++Cd��
�ʴ�Ϊ��Zn+Cd2+=Zn2++Cd��
��5��Ҫ����Һ�еõ�����п���壬Ӧ����ͨ������Ũ���������ᾧ��Ȼ����ˣ�ϴ�Ӽ��ɣ�Ϊ��ֹ����пˮ����������ʣ�Ӧ����һ������ȣ��ʴ�Ϊ������һ������ȣ�

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ