ƒøƒ⁄»›
N2O 5∫‹≤ªŒ»∂®£¨‘⁄t °Ê ±”–œ¬¡–¡Ω∏ˆø…ƒÊ∑¥”¶£∫N2O5£®g£©£®1£©∆Ω∫‚ ±N2O5£®g£©∫ÕN2O£®g£©µƒ≈®∂»°£
£®2£©¥”∑¥”¶ø™ ºµΩ¥ÔµΩ∆Ω∫‚ ±N2O5£®g£©µƒ∆Ωæ˘∑¥”¶ÀŸ¬ °£
£®1£©c(N2O5)=0.009 4 mol°§L-1£¨c(N2O)=0.014 4 mol°§L-1
£®2£©v(N2O5)=0.006 12 mol°§L-1°§min-1
Ω‚Œˆ£∫…Ë¥ÔµΩ∆Ω∫‚ ±œ˚∫ƒµƒN2O5£®g£©ŒÔ÷ µƒ¡ø≈®∂»Œ™x£¨…˙≥…µƒN2O£®g£©ŒÔ÷ µƒ¡ø≈®∂»Œ™y£¨‘Ú
N2O5£®g£© ![]() N2O3(g) + O2(g)
N2O3(g) + O2(g)
ø™ º£∫0.040 0 mol°§L-1 0 0
∆Ω∫‚£∫0.040 0 mol°§L-1-x x x
N2O3(g)![]() N2O(g)+O2(g)
N2O(g)+O2(g)
∆Ω∫‚£∫x-y y y
“¿Ã‚“‚µ√£∫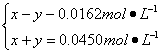
Ω‚µ√
À˘“‘£¨£®1£©∆Ω∫‚ ±c(N2O5)=0.040 0 mol°§L-1-0.030 6 mol°§L-1=0.009 4 mol°§L-1,c(N2O)=0.014 4 mol°§L-1°£
![]()

¡∑œ∞≤·œµ¡–¥∞∏
œ‡πÿƒø