��Ŀ����
��8�֣�ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.00 mL����ƿ�У�����10.00 mL KI��Һ(����)������ָʾ��2��3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.01 mol��L-1Na2S2O3��Һ���Լ��ԣ�������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2 + 2Na2S2O3 =" 2NaI" + Na2S4O6��
������������⣺
(1)����ټ����ָʾ����____________________��
(2)�����Ӧʹ��____________ʽ�ζ��ܡ�
(3)����۵�����Һ��___________ɫ��Ϊ__________ɫ�Ҳ��ٱ仯�����յ㣬����ȥNa2S2O3��Һ40.00 mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ__________________________��
(4)����ʵ�鲽������һ�����ԵIJ�������,��ָ��.
��ȡˮ��10.00 mL����ƿ�У�����10.00 mL KI��Һ(����)������ָʾ��2��3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.01 mol��L-1Na2S2O3��Һ���Լ��ԣ�������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2 + 2Na2S2O3 =" 2NaI" + Na2S4O6��
������������⣺
(1)����ټ����ָʾ����____________________��
(2)�����Ӧʹ��____________ʽ�ζ��ܡ�
(3)����۵�����Һ��___________ɫ��Ϊ__________ɫ�Ҳ��ٱ仯�����յ㣬����ȥNa2S2O3��Һ40.00 mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ__________________________��
(4)����ʵ�鲽������һ�����ԵIJ�������,��ָ��.

��

��ϰ��ϵ�д�
�����Ŀ
 ����___________ (�����)��Һ����Ӧ�����ӷ���ʽΪ______________________��
����___________ (�����)��Һ����Ӧ�����ӷ���ʽΪ______________________��
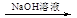 ���ɫ����
���ɫ����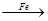 FeBr3
FeBr3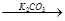 KBr
KBr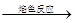 ��ɫ�ʻ�ɫ
��ɫ�ʻ�ɫ
 ���¿̶ȡ�
���¿̶ȡ� Щʵ��
Щʵ�� ��������� ��
��������� �� __________________________________________________
__________________________________________________ ______________________��
______________________�� 1)����Һ����ȡ20.00 mL FeSO4��Һ������ƿ�У���0.10 mol��L��1������KMnO4��Һ�����յ㣬��ȥKMnO4��Һ20.00 mL������������MnԪ��ȫ���ʣ�2�ۣ��ζ���Ӧ�����ӷ���ʽΪ______ __���ݴ˿ɲ��FeSO4��Һ�����ʵ���Ũ��Ϊ______ __mol��L��1��
1)����Һ����ȡ20.00 mL FeSO4��Һ������ƿ�У���0.10 mol��L��1������KMnO4��Һ�����յ㣬��ȥKMnO4��Һ20.00 mL������������MnԪ��ȫ���ʣ�2�ۣ��ζ���Ӧ�����ӷ���ʽΪ______ __���ݴ˿ɲ��FeSO4��Һ�����ʵ���Ũ��Ϊ______ __mol��L��1��