��Ŀ����
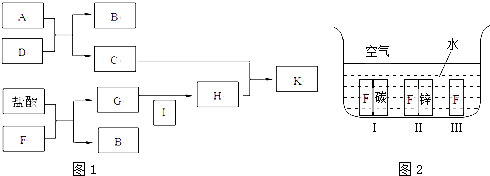
A��K��Ϊ��ѧ��ѧ�г������ʣ���ת����ϵ��ͼ��ʾ��AΪ���������DΪ����ɫ��ĩ��KΪ��ɫ��״������ͼ�в��ַ�Ӧ��������Ӧ������δע����������д���пհף�
��1��д���������ʵĻ�ѧʽA ��D ��
��2��C��D�Ļ����ͨ���� ��
��3��д�����л�ѧ����ʽ�����ӷ���ʽ��
A��J �����ӷ���ʽ�� ��
G+B��H ����ѧ����ʽ�� ��
��4��I����Һͨ�������ԣ��������ӷ���ʽ����ԭ�� ��

��1��д���������ʵĻ�ѧʽA
��2��C��D�Ļ����ͨ����
��3��д�����л�ѧ����ʽ�����ӷ���ʽ��
A��J �����ӷ���ʽ��
G+B��H ����ѧ����ʽ��
��4��I����Һͨ�������ԣ��������ӷ���ʽ����ԭ��
����������ת����ϵ�������������ʵ������ͷ�Ӧ�����жϣ�AΪ���������DΪ����ɫ��ĩ��KΪ��ɫ��״����������ת����ϵ��A���ᡢ�Ӧ˵�������������ΪAl2O3��IΪAlCl3��JΪNaAlO2��KΪAl��OH��3�����������ڵ����������������������֪BΪO2��CΪAl��DΪFe2O3��EΪ���ȷ�Ӧ���ɵĽ���Fe��FΪFeCl2��GΪFe��OH��2��HΪFe��OH��3�������ƶ����ʻش����⣮
����⣺����ת����ϵ�������������ʵ������ͷ�Ӧ�����жϣ�AΪ���������DΪ����ɫ��ĩ��KΪ��ɫ��״����������ת����ϵ��A���ᡢ�Ӧ˵�������������ΪAl2O3��IΪAlCl3��JΪNaAlO2��KΪAl��OH��3�����������ڵ����������������������֪BΪO2��CΪAl��DΪFe2O3��EΪ���ȷ�Ӧ���ɵĽ���Fe��FΪFeCl2��GΪFe��OH��2��HΪFe��OH��3��
��1��AΪ����������ѧʽΪ��Al2O3��DΪ����������ѧʽΪ��Fe2O3���ʴ�Ϊ��Al2O3��Fe2O3��
��2��������֪CΪAl��DΪFe2O3��������Ƴ����ȼ����ʴ�Ϊ�����ȼ���
��3��A��J ��������������������Һ�ķ�Ӧ����Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O��G+B��H ������������������ˮ�����ķ�Ӧ����������������Ӧ�Ļ�ѧ����ʽ4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��4��IΪ�Ȼ�����Һ����Һ��������ˮ�������ԣ���Һ�����Ե����ӷ���ʽΪ��Al3++3H2O?Al��OH��3+3H+���ʴ�Ϊ��Al3++3H2O?Al��OH��3+3H+��
��1��AΪ����������ѧʽΪ��Al2O3��DΪ����������ѧʽΪ��Fe2O3���ʴ�Ϊ��Al2O3��Fe2O3��
��2��������֪CΪAl��DΪFe2O3��������Ƴ����ȼ����ʴ�Ϊ�����ȼ���
��3��A��J ��������������������Һ�ķ�Ӧ����Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O��G+B��H ������������������ˮ�����ķ�Ӧ����������������Ӧ�Ļ�ѧ����ʽ4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��4��IΪ�Ȼ�����Һ����Һ��������ˮ�������ԣ���Һ�����Ե����ӷ���ʽΪ��Al3++3H2O?Al��OH��3+3H+���ʴ�Ϊ��Al3++3H2O?Al��OH��3+3H+��
���������⿼��������ת����ϵ���������ʵķ����жϣ���Ҫ�������仯��������仯�������ʵķ���Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ