��Ŀ����
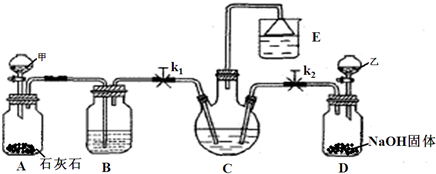
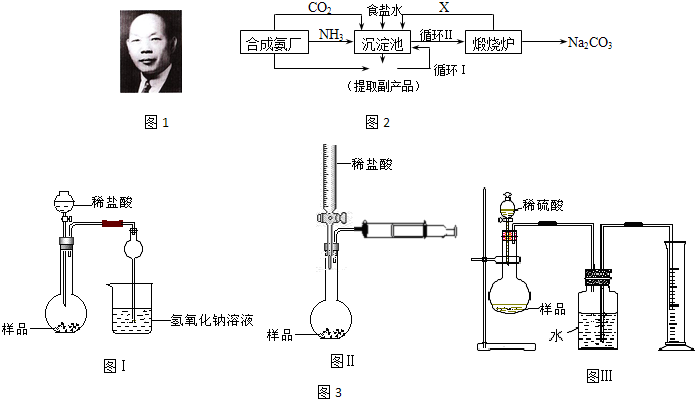
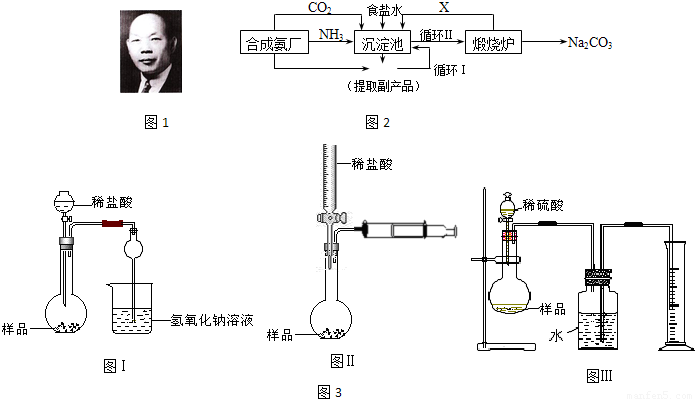
�ҹ���ѧ�Һ�°�ͼ1���ĸ����Ĵ����������գ��������̿ɼ�Ҫ��ʾ��ͼ2��
��1��д������������X���ʵķ���ʽ
��2��ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%���ϣ���Ҫ�漰��
��3����ʵ��õ��Ĵ����к����Ȼ��ƣ���ͬѧ�����ͼ3װ�â���ͨ���ⶨ�ձ�������������Һ���������ⶨ��Ʒ��̼���Ƶĺ���������ʵ��������Բ��㣬�Ծٳ���װ����������������Ҫԭ��
��
��
��4����ͬѧ��ƵIJⶨ��Ʒ�д�����ķ�����ͼ3���Իش�
�ټ���װ�������Եķ���Ϊ��
����ζ��ܵ���ʼ����ΪV1mL�����˶���ΪV2mL��ע�����ռ�������ΪV3mL����״�������Ƶ���Ʒ����Ϊm g����ԭ��Ʒ��̼���Ƶ����������ı���ʽΪ
��5����ͬѧ��ƵIJⶨ��Ʒ�д�����ķ�����ͼ3���ڹ��ƿ�е�ˮ����μ�����ֲ���ͣ��Իش�
������Ϊֲ���͵�������
��Ϊ��С����ȡ��Ͳ��ˮ�����ʱ��ע�������У�

��1��д������������X���ʵķ���ʽ
CO2
CO2
���������з����Ļ�ѧ��Ӧ����ʽ��NH3+H2O+CO2+NaCl��NH4Cl+NaHCO3��
NH3+H2O+CO2+NaCl��NH4Cl+NaHCO3��
����2��ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%���ϣ���Ҫ�漰��
ѭ����
ѭ����
�������������еı�ţ���ѭ�����ӳ�������ȡ�������IJ���������
����
����3����ʵ��õ��Ĵ����к����Ȼ��ƣ���ͬѧ�����ͼ3װ�â���ͨ���ⶨ�ձ�������������Һ���������ⶨ��Ʒ��̼���Ƶĺ���������ʵ��������Բ��㣬�Ծٳ���װ����������������Ҫԭ��
��
ϡ����ӷ������Ȼ�������Ҳ������������Һ����
ϡ����ӷ������Ȼ�������Ҳ������������Һ����
����
��ƿ�͵����������Ķ�����̼���岻�ܱ�����������Һ����
��ƿ�͵����������Ķ�����̼���岻�ܱ�����������Һ����
�� ��4����ͬѧ��ƵIJⶨ��Ʒ�д�����ķ�����ͼ3���Իش�
�ټ���װ�������Եķ���Ϊ��
�ر���ʽ�ζ��ܻ���������������ƿ����ע�����������ƣ����ֺ�ص�ԭ��λ�ã�˵������������
�ر���ʽ�ζ��ܻ���������������ƿ����ע�����������ƣ����ֺ�ص�ԭ��λ�ã�˵������������
������ζ��ܵ���ʼ����ΪV1mL�����˶���ΪV2mL��ע�����ռ�������ΪV3mL����״�������Ƶ���Ʒ����Ϊm g����ԭ��Ʒ��̼���Ƶ����������ı���ʽΪ
106��
��100%
| V3-(V2-V1) |
| 22400m |
106��
��100%
���ú�V1��V2��V3��m�Ĵ���ʽ��ʾ��| V3-(V2-V1) |
| 22400m |
��5����ͬѧ��ƵIJⶨ��Ʒ�д�����ķ�����ͼ3���ڹ��ƿ�е�ˮ����μ�����ֲ���ͣ��Իش�
������Ϊֲ���͵�������
���ٶ�����̼��ˮ�е��ܽ⣬��Сʵ�����
���ٶ�����̼��ˮ�е��ܽ⣬��Сʵ�����
����Ϊ��С����ȡ��Ͳ��ˮ�����ʱ��ע�������У�
����ǰ�����ƶ���Ͳʹ���ƿ��Һ������Ͳ��Һ����ƽ����ȡ��Ͳ��ˮ�����ʱҪƽ��
����ǰ�����ƶ���Ͳʹ���ƿ��Һ������Ͳ��Һ����ƽ����ȡ��Ͳ��ˮ�����ʱҪƽ��
����������1��������̼�Ƿ�Ӧ��ԭ��ͬʱҲ�Ƿ�Ӧ�ĸ��������ѭ�����ã������Ļ�ѧ��ӦΪ����ʳ��ˮ��ͨ�백���Ͷ�����̼����̼�����ƾ��壻
��2��ѭ�����е��Ȼ������ַ��ص������أ�
��3����ϡ����Ļӷ��������Ķ�����̼���ܱ�����������ȫ���շ�����
��4���ٹرյζ��ܻ���������������ƿ��ͨ���۲�ע���������ı仯����жϣ�
���ҳ�������̼������������̼���Ƶ����ʵ����������������ɣ�
��5���ٴӶ�����̼���ܽ�ȿ��ǣ�������̼������ֲ���ͣ�
��ʹ���ƿ��Һ������Ͳ��Һ����ƽʱ����������ȷ������ʱ������Ҫ����Ͳ��ˮ�İ�Һ����ƽ��
��2��ѭ�����е��Ȼ������ַ��ص������أ�
��3����ϡ����Ļӷ��������Ķ�����̼���ܱ�����������ȫ���շ�����
��4���ٹرյζ��ܻ���������������ƿ��ͨ���۲�ע���������ı仯����жϣ�
���ҳ�������̼������������̼���Ƶ����ʵ����������������ɣ�
��5���ٴӶ�����̼���ܽ�ȿ��ǣ�������̼������ֲ���ͣ�
��ʹ���ƿ��Һ������Ͳ��Һ����ƽʱ����������ȷ������ʱ������Ҫ����Ͳ��ˮ�İ�Һ����ƽ��
����⣺��1�������������Ƽ�ж�����̼�Ƿ�Ӧ��ԭ��ͬʱҲ�Ƿ�Ӧ�ĸ��������ѭ�����ã�
�������з����Ļ�ѧ��ӦΪ����ʳ��ˮ��ͨ�백���Ͷ�����̼����̼�����ƾ��壬��Ӧ����ʽΪNH3+H2O+CO2+NaCl��NH4Cl+NaHCO3����
�ʴ�Ϊ��CO2��NH3+H2O+CO2+NaCl��NH4Cl+NaHCO3����
��2��ѭ�����ǽ�δ��Ӧ���Ȼ��Ʒ��س������У��ӹ�Һ������з��������ķ���Ϊ���ˣ�
�ʴ�Ϊ��ѭ�����ˣ�
��3����װ����������������Ҫԭ���У���ϡ����ӷ������Ȼ�������Ҳ������������Һ���գ� ����ƿ�͵����������Ķ�����̼���岻�ܱ�����������Һ���գ�
�ʴ��ǣ�ϡ����ӷ������Ȼ�������Ҳ������������Һ���գ���ƿ�͵����������Ķ�����̼���岻�ܱ�����������Һ���գ�
��4���ټ���װ�������Եķ���Ϊ���ر���ʽ�ζ��ܻ���������������ƿ����ע�����������ƣ����ֺ�ص�ԭ��λ�ã�˵�����������ã�
�ʴ��ǣ��ر���ʽ�ζ��ܻ���������������ƿ����ע�����������ƣ����ֺ�ص�ԭ��λ�ã�˵�����������ã�
�������ų��Ŀ���������ǣ�V2-V1��mL�������Ķ�����̼�������V3-��V2-V1��������̼ԭ���غ㣬̼���Ƶ����ʵ����ǣ�
mol��
ԭ��Ʒ��̼���Ƶ���������Ϊ��106��
��100%��
�ʴ��ǣ�106��
��100%��
��5����ֲ���͵������Ǽ��ٶ�����̼��ˮ�е��ܽ⣬��Сʵ����
�ʴ��ǣ����ٶ�����̼��ˮ�е��ܽ⣬��Сʵ����
��Ϊ��С����ȡ��Ͳ��ˮ�����ʱ��ע�������У�����ǰ�����ƶ���Ͳʹ���ƿ��Һ������Ͳ��Һ����ƽ����ȡ��Ͳ��ˮ�����ʱҪƽ�ӣ�
����ǰ�����ƶ���Ͳʹ���ƿ��Һ������Ͳ��Һ����ƽ����ȡ��Ͳ��ˮ�����ʱҪƽ�ӣ�
�������з����Ļ�ѧ��ӦΪ����ʳ��ˮ��ͨ�백���Ͷ�����̼����̼�����ƾ��壬��Ӧ����ʽΪNH3+H2O+CO2+NaCl��NH4Cl+NaHCO3����
�ʴ�Ϊ��CO2��NH3+H2O+CO2+NaCl��NH4Cl+NaHCO3����
��2��ѭ�����ǽ�δ��Ӧ���Ȼ��Ʒ��س������У��ӹ�Һ������з��������ķ���Ϊ���ˣ�
�ʴ�Ϊ��ѭ�����ˣ�
��3����װ����������������Ҫԭ���У���ϡ����ӷ������Ȼ�������Ҳ������������Һ���գ� ����ƿ�͵����������Ķ�����̼���岻�ܱ�����������Һ���գ�
�ʴ��ǣ�ϡ����ӷ������Ȼ�������Ҳ������������Һ���գ���ƿ�͵����������Ķ�����̼���岻�ܱ�����������Һ���գ�
��4���ټ���װ�������Եķ���Ϊ���ر���ʽ�ζ��ܻ���������������ƿ����ע�����������ƣ����ֺ�ص�ԭ��λ�ã�˵�����������ã�
�ʴ��ǣ��ر���ʽ�ζ��ܻ���������������ƿ����ע�����������ƣ����ֺ�ص�ԭ��λ�ã�˵�����������ã�
�������ų��Ŀ���������ǣ�V2-V1��mL�������Ķ�����̼�������V3-��V2-V1��������̼ԭ���غ㣬̼���Ƶ����ʵ����ǣ�
| V3-(V2-V1) |
| 22400 |
ԭ��Ʒ��̼���Ƶ���������Ϊ��106��
| V3-(V2-V1) |
| 22400m |
�ʴ��ǣ�106��
| V3-(V2-V1) |
| 22400m |
��5����ֲ���͵������Ǽ��ٶ�����̼��ˮ�е��ܽ⣬��Сʵ����
�ʴ��ǣ����ٶ�����̼��ˮ�е��ܽ⣬��Сʵ����
��Ϊ��С����ȡ��Ͳ��ˮ�����ʱ��ע�������У�����ǰ�����ƶ���Ͳʹ���ƿ��Һ������Ͳ��Һ����ƽ����ȡ��Ͳ��ˮ�����ʱҪƽ�ӣ�
����ǰ�����ƶ���Ͳʹ���ƿ��Һ������Ͳ��Һ����ƽ����ȡ��Ͳ��ˮ�����ʱҪƽ�ӣ�
���������⿼���˺����Ƽ��Ӧ�Լ�������Ļ������ã��漰�˻�ѧ����ʽ����д�����������ļ����֪ʶ�����ݽ϶࣬�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ