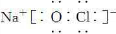��Ŀ����
��ͼ��A��D����ѧ��ѧʵ���г����ļ����¶ȼ�װ��ʾ��ͼ.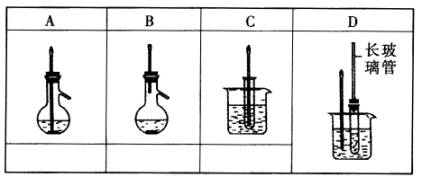
(1)�����������ѡ������ʹ���¶ȼƵ�ʵ�飬�ѱ�����������˵�װ��ͼA��C�µĿո���(��ѡҪ���۷�).
���ƾ���Ũ�����ϼ�������ϩ
����ʯ��ˮ��Ӧ����Ȳ
�����뱽���������Ļ����
���������ȡ����Ӧ
��ʯ�ͷ���ʵ��
��Ũ����Ͷ������̻�ϼ���������
���ⶨ�������ˮ�е��ܽ��
��ʳ�κ�Ũ�����ϼ������Ȼ���
(2)ѡ��װ��D����������ʵ�飬D�г������ܵ�������________.
�𰸣�
������
��ʾ��
������
| (1)A�� B�ۢ� C��
(2)���ٱ��Ļӷ�(�����������������)
|
��ʾ��
| �ڵ�(1)�����о���8��ѡ�����ʵ��A��B������������ƿ��ǰ�������¶ȼƲ�����ӦҺ���¶ȣ�ѡ����ֻ���Ǣ٣����������¶ȼƲ����������¶ȣ����������ָ������֣��ۺ͢ݶ���������ʵ��.װ��C��������ʵ��ֻ��ѡ��߿��Բ���.
�ڵ�(2)�������漰�ı������������ڱ���������ӦҪ��50�桫60��ʱ���У����ҷ�Ӧ�����һ��ʱ�䣬����ӷ��ı����������������ʱ�С��Ĵ�Ӧ�ǡ����ٱ��Ļӷ�(�������������) ��.
|

��ϰ��ϵ�д�
�����Ŀ