��Ŀ����
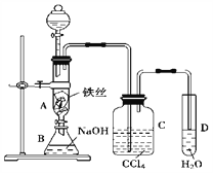
����Ŀ��ijУ��ѧ��ȤС��Ϊ�о�Cl2�����ʣ������ͼ��ʾװ�ý���ʵ��
(1)ʵ�����Զ������̺�Ũ�����Ʊ������Ļ�ѧ����ʽΪ_______________��
(2)װ�â��������____________________________________��
(3)ʵ������У�װ�â��е�ʵ������Ϊ_________________________��������Ӧ�Ļ�ѧ����ʽΪ_______________________________��
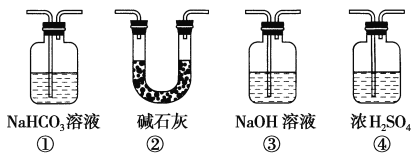
(4)ʵ���������ͬѧ��װ�â���(a�Ǹ����Ʒ����ֽ��b�dz�ʪ��Ʒ����ֽ)�۲쵽b�ĺ�ɫ��ȥ�����Dz�δ�۲쵽��a�����Ա仯����һԤ������Ϊ�˴ﵽ��һʵ��Ŀ�ģ�����Ϊ��������ͼװ��_______��_____________֮��������ͼ�е�_____________װ��(�����)����װ�õ�������____________��
(5)װ��V��Ŀ���Ƿ�ֹβ����Ⱦ������д��װ��V�з�����Ӧ�Ļ�ѧ����ʽ��_________________��
(6)��8.7 g MnO2�뺬HCl 14.6 g��Ũ���Ṳ����Cl2����ͬѧ��Ϊ���Ƶ�Cl2 7.1 g����ͬѧ��Ϊ�Ƶ�Cl2������С��7.1 g������Ϊ__________(����������������)ͬѧ��ȷ��ԭ���� ___________________��
���𰸡�MnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O ��ȥ�����е��Ȼ������� ��ɫ��Һ����ɫ Cl2��2KI===I2��2KCl �� �� �� ����Cl2 Cl2��2NaOH===NaCl��NaClO��H2O �� ���ŷ�Ӧ���У�Ũ����Ũ�Ȼή�ͣ�ϡ������MnO2����Ӧ
MnCl2+Cl2��+2H2O ��ȥ�����е��Ȼ������� ��ɫ��Һ����ɫ Cl2��2KI===I2��2KCl �� �� �� ����Cl2 Cl2��2NaOH===NaCl��NaClO��H2O �� ���ŷ�Ӧ���У�Ũ����Ũ�Ȼή�ͣ�ϡ������MnO2����Ӧ
��������
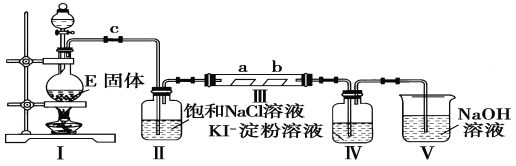
������ʵ�����Ʊ�Cl2����֤Cl2ijЩ���ʵ�ʵ�顣װ��I��ʵ������Cl2��װ�ã�װ�б���ʳ��ˮ��ϴ��ƿ��Ϊ�˳�ȥCl2�е�HCl���壬װ��III�Ǽ�����ˮ��Ư���ԣ�ͬʱ��֤Cl2��Ư���ԣ�IVװ����֤Cl2�������ԣ����װ��V���ն����Cl2����ֹ��Ⱦ������
(1)ʵ�����ö������̺�Ũ���Ṳ���Ʊ��������仯ѧ����ʽΪMnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O��
(2)Ũ�����ܻӷ���HCl���壬�Ƶõ������л����HCl���壬��Cl2�ڱ���ʳ��ˮ���ܽ����С��HCl�����ּ�������ˮ������װ��II�������dz�ȥ�����е��Ȼ������塣
(3)��ΪCl2�������Դ���I2��Cl2ͨ����ɫ��KI��Һ�з�����Ӧ��2KI+Cl2=I2+2KCl��I2��ʹ���۱���������װ��IV�е�ʵ������Ϊ��ɫ��Һ����ɫ��������Ӧ�Ļ�ѧ����ʽΪ2KI+Cl2=I2+2KCl��
(4)����ͨ������ʳ��ˮʱ�����ˮ��������ʪ��Cl2�л�����HClO��HClO����Ư���ԣ�����a�������Ʒ����ֽ��ɫ����ʹ��a�����Ա仯�������������������װ��IIIǰ��ȥ�����е�ˮ����������Ҫ��װ��II��װ��III֮������һ����װ�ã�����ѡ�����������Cl2��Ӧ��NaHCO3��Һ����ʯ�ҡ�NaOH��Һ������Cl2��Ӧ������ֻ��ѡ��װ���ܣ�����װ��II��װ��III֮�䡣
(5)Cl2�ж���������NaOH��Ӧ����NaOH��ҺŨ�Ƚϴ����ܳ�����ն����Cl2����ֹCl2��Ⱦ�������䷢����Ӧ�Ļ�ѧ����ʽ��Cl2+2NaOH=NaCl+NaClO+H2O��
(6)8.7gMnO2�����ʵ���=![]() =0.1mol��14.6gHCl�����ʵ���=
=0.1mol��14.6gHCl�����ʵ���=![]() =0.4mol�����ݷ�Ӧ����ʽMnO2+4HCl(Ũ)
=0.4mol�����ݷ�Ӧ����ʽMnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O��֪��ֻ��0.1molMnO2��0.4molHClǡ����ȫ��Ӧ���ܲ���0.1molCl2����7.1gCl2�������ǣ���Ӧ����������HCl�������Լ���Ӧ����ˮ��ʹ��Ũ������ϡ����ϡ����ܸ�MnO2��Ӧ�����0.1molMnO2��0.4molHCl������ȫ��Ӧ�����Բ�����Cl2С��0.1mol����������Cl2����С��7.1g������ͬѧ��ȷ��
MnCl2+Cl2��+2H2O��֪��ֻ��0.1molMnO2��0.4molHClǡ����ȫ��Ӧ���ܲ���0.1molCl2����7.1gCl2�������ǣ���Ӧ����������HCl�������Լ���Ӧ����ˮ��ʹ��Ũ������ϡ����ϡ����ܸ�MnO2��Ӧ�����0.1molMnO2��0.4molHCl������ȫ��Ӧ�����Բ�����Cl2С��0.1mol����������Cl2����С��7.1g������ͬѧ��ȷ��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�