��Ŀ����
11���±���Ԫ�����ڱ���һ���֣�������ĸ�ֱ����һ��Ԫ�أ�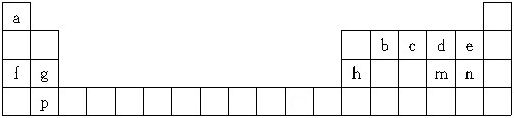
��1��mԪ�������ڱ��е�λ���ǵ������ڢ�A�壮
��2�������й�˵����ȷ����ABC������ĸ����
A��b��c��dԪ�صķǽ�����������
B��f��g��hԪ�ص�ԭ�Ӱ뾶��С
C��md2��bd2�Ļ�ѧ�������ƣ�������������
D��e��n����ۺ����������ǿ����e��n
E��a��f�ֱ���d��ɵĻ�������������ѧ��������ȫ��ͬ
F���ñ���ֻ��4��Ԫ����ɵĵ��ʾ��е�����
��3��a��c��n��ԭ�Ӹ�����Ϊ4��1��1���ɵĻ�����ĵ���ʽ��
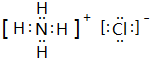 ��
����4��P4�����ף������ʯ��MgCl2���־����۵��ɸߵ��͵�˳��Ϊ���ʯ��MgCl2��P4�����ף���
���� ��Ԫ�������ڱ���λ�ã���֪aΪH��bΪC��cΪN��dΪO��eΪF��fΪNa��gΪMg��hΪAl��mΪS��nΪCl��pΪCa��
��1����mԪ�������ڱ��е�λ�ã���֪��λ�ڵ������ڢ�A�壻
��2��A��ͬ����������ҷǽ�������ǿ��
B��ͬ������ԭ����������ԭ�Ӱ뾶��С��
C��SO2��CO2��Ϊ�������������������SԪ��Ϊ�м��̬�����������ԣ�������̼��CԪ��Ϊ��ۣ����������ԣ�
D����Ԫ��û����ۺ����
E��a��d ��ɵĻ�����ΪH2O��H2O2��f��d��ɵĻ�����ΪNa2O��Na2O2��
F���������ʿ��Ե��磬ʯīҲ���Ե��磻
��3��a��c��n��ԭ�Ӹ�����Ϊ4��1��1���ɵĻ�����ΪNH4Cl����笠������������ӹ��ɣ�
��4���۷е�һ���ǣ�ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壮
��� �⣺��Ԫ�������ڱ���λ�ã���֪aΪH��bΪC��cΪN��dΪO��eΪF��fΪNa��gΪMg��hΪAl��mΪS��nΪCl��pΪCa��
��1����mԪ�������ڱ��е�λ�ã���֪��λ�ڵ������ڢ�A�壬
�ʴ�Ϊ���������ڢ�A�壻
��2��A��ͬ����������ҷǽ�������ǿ����A��ȷ��
B��ͬ������ԭ����������ԭ�Ӱ뾶��С����B��ȷ��
C��SO2��CO2��Ϊ�������������������SԪ��Ϊ�м��̬�����������ԣ�������̼��CԪ��Ϊ��ۣ����������ԣ���C��ȷ��
D����Ԫ��û����ۺ����ᣬ��D����
E��a��d ��ɵĻ�����ΪH2O��H2O2�����й��ۼ���f��d��ɵĻ�����ΪNa2O��Na2O2�����������Ӽ����������й��ۼ�����E����
F���������ʿ��Ե��磬ʯīҲ���Ե��磬������5��Ԫ����ɵĵ��ʾ��е����ԣ���F����
��ѡ��ABC��
��3��a��c��n��ԭ�Ӹ�����Ϊ4��1��1���ɵĻ�����ΪNH4Cl����笠������������ӹ��ɣ�����ʽΪ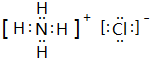 ��
��
�ʴ�Ϊ��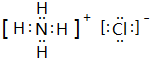 ��
��
��4�����ʯ����ԭ�Ӿ��壬MgCl2�������Ӿ��壬P4�����ף����ڷ��Ӿ��壬���۵㣺���ʯ��MgCl2��P4�����ף���
�ʴ�Ϊ�����ʯ��MgCl2��P4�����ף���
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã���Ҫѧ����������Ԫ�����ڱ�����2����DFѡ��Ϊ�״��㣬ѧ�������ԡ�FԪ��û����ۺ����ᡢʯī���Ե��硱��

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�| A�� | �ǻ��ĵ���ʽ�� | B�� | ��������ģ��Ϊ�� | ||
| C�� |  �� �� ��Ϊͬϵ�� ��Ϊͬϵ�� | D�� | ���ǻ�������Ľṹ��ʽ�� |
| A�� | 5 | B�� | 6 | C�� | 7 | D�� | 8 |
| A�� | ��0.1mol/LNaHCO3��Һ�У�c��Na+����c��HCO3-����C��CO32-����c��H2CO3�� | |
| B�� | ��0.1mol/LNa2CO3��Һ�У�c��OH-��-c��H+��=c��HC03-��+c��H2CO3�� | |
| C�� | ��0.2mol/LNaHCO3��Һ�м�������0.1mol/LNaOH��Һ��c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| D�� | �����£�CH3COONa��CH3COOH�����Һ[pH=7��c��Na+��=0.1mol/L]��c��Na+��=c��CH3COO-����c��CH3COOH����c��H+��=c��OH-�� |
| A�� | �ڢ�A��Ԫ�ض��ǵ��͵Ľ���Ԫ�� | |
| B�� | �������ڵ�Ԫ�ص�ԭ�Ӻ��ⶼ���������Ӳ� | |
| C�� | F��Cl��O��N����Ԫ�ض��ǵڢ�A���Ԫ�� | |
| D�� | ԭ�ӵ���������������ӵ�Ԫ�ض��ڵڢ�A�� |

| A�� | X����������ʯī | B�� | ���Ӵ�ͭ�缫�����·����X�缫 | ||
| C�� | Y������ͭ��Һ | D�� | X���ϵĵ缫��ӦʽΪAg++e-�TAg |


 ����������ѧ��Ӧԭ��֪ʶ����������⣺
����������ѧ��Ӧԭ��֪ʶ����������⣺