��Ŀ����
��C1��ѧ����ָ�Է�����ֻ��һ��̼ԭ�ӵ�����Ϊԭ�Ͻ������ʺϳɵĻ�ѧ����C1��ѧ�����ڻ������� ���ص���ԴΣ������������ú����Ȼ���Ȼ�ʯȼ�ϡ����������ȶ��зdz���Ҫ�����塣�ϳ���(CO+H2)�� ��C1��ѧ���еij���ԭ�ϡ�
(1)ú���������ɺϳ������÷�Ӧ�Ļ�ѧ����ʽΪ____________________���ø÷��������ϳ�����һ������ȱ����___________��
(2)�������������ƺϳ�����CH4(g)+1/2O2(g) CO(g)+2H2(g) ��H=-35.6 kJ/mol���÷�Ӧ��_____����Է������Է�������Ӧ��
CO(g)+2H2(g) ��H=-35.6 kJ/mol���÷�Ӧ��_____����Է������Է�������Ӧ��
(3)ͨ���Ҵ���ȡ�ϳ����������õ�Ӧ��ǰ�������Ҵ���ȡ�ϳ�������������·�ߣ�
a��ˮ������������CH3CH2OH(g)+H2O(g)��4H2(g)+2CO(g) ��H=+255.58 kJ/mol
b�����ִ�������CH3CH2OH(g)+1/2O2(g)�� 3H2(g)+2CO(g) ��H=+13.76 kJ/mol
����˵���������____��
A����ԭ�����ĵĽǶ�������a·��������м�ֵ
B�����������ĵĽǶ�������b·�������������
C��a·����������Ҫ���ĺܶ�������������ʵ�����������岻��
D��������������Ӧ�У�ԭ�������ʽϸߵ���b��Ӧ
(4)��ҵ�úϳ����Ʊ������ѵ������������£�

CO(g)+2H2(g)
 CH3OH(g) ��H=-90.7 kJ/mol ��
CH3OH(g) ��H=-90.7 kJ/mol ��2CH3OH(g)
 CH3OCH3(g)+H2O(g) ��H = -23.5 kJ/mol ��
CH3OCH3(g)+H2O(g) ��H = -23.5 kJ/mol ��CO(g) +H2O(g)
 CO2(g)+H2(g) ��H=-41.2 kJ/mol ��
CO2(g)+H2(g) ��H=-41.2 kJ/mol �� �ٴ���Ӧ�����ܷ�Ӧ3CO(g)+3H2(g)
 CH3OCH3(g)+CO2(g)�ġ�H=_____��830�� ʱ��Ӧ�۵�K=1.0�����ڴ���Ӧ���з�Ӧ�۵�K_______���>������<����=����1.0��
CH3OCH3(g)+CO2(g)�ġ�H=_____��830�� ʱ��Ӧ�۵�K=1.0�����ڴ���Ӧ���з�Ӧ�۵�K_______���>������<����=����1.0�� �����������У�����ѭ��ʹ�õ�������____________��
 CO+H2���ܺĴ�
CO+H2���ܺĴ�(2)�Է�
(3)D
(4)��-246.1 kJ/mol��>����CO��H2���״���ˮ

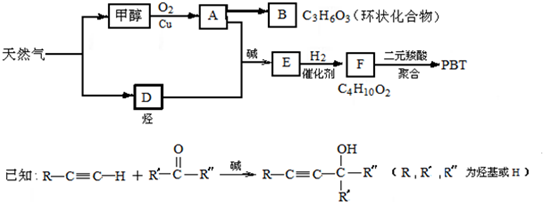
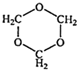

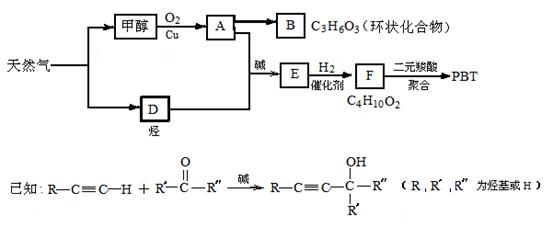
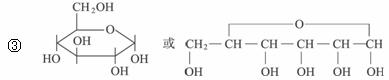
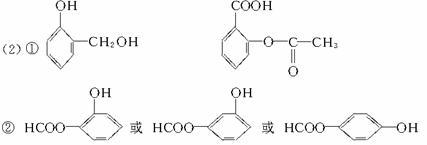 д������A�Ļ�ѧ����ʽ��_________________________________��
д������A�Ļ�ѧ����ʽ��_________________________________��