��Ŀ����
N��S����ѧ��ѧ����Ҫ�ķǽ���Ԫ�أ��ش��������⣺
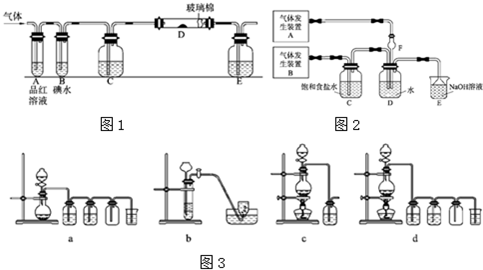
��1��ʵ��������Cu�ֱ��Ũ���ᡢŨ���ᷴӦ��ȡ���ռ�SO2��NO2�����߶���Ҫ���������е�__________(����)

��2����1molNaOH����Һ��ͨ��1molSO2ǡ����ȫ��Ӧ�����ɵ���Ϊ___________����ʱ����Һ�����ԣ���ԭ����__________________________________________��
��3��Һ����ijЩ������ˮ���ơ���֪ˮ���������ܷ�����ż���룬���ɵ����������Ӿ�����10�����ӡ�д��Һ�����ӷ�����ż����ķ���ʽ____________________��
Һ����Na��Ӧ�Ļ�ѧ����ʽΪ������������������ ��
��4����ˮϡ��0.1 mol��L��1��ˮʱ������ˮ�������Ӷ��������_______������ţ�
a��![]() ���������������������� b��
���������������������� b��![]()
c��![]() ������������������������ d��
������������������������ d��![]()
��5����(NH4)2SO4��ǿ��ʱ�ֽ�IJ�����SO2��N2��NH3��H2O����ÿ����1mol N2��ͬʱ���������������� mol SO2��
��1��B C F����2������ȷ��1�֣�ȫ�Ե�2�֣�
��2��NaHSO3��2�֣���HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�������Һ�����ԡ���2�֣�
��3��2NH3![]() NH4++NH2- ��2�֣� 2Na+2NH3===2NaNH2+ H2����2�֣�
NH4++NH2- ��2�֣� 2Na+2NH3===2NaNH2+ H2����2�֣�
��4��a c��ֻ��һ������ȷ��1�֣�
��5��3��2�֣�