ЬтФПФкШн
ЁОЬтФПЁПФПЧАЪРНчЩЯЙуЗКВЩгУАБКЭЖўбѕЛЏЬМИпЮТИпбЙЯТжЦБИФђЫиЃЌжївЊЗДгІЮЊСНВНЃК
ЕквЛВНЃКЩњГЩАБЛљМзЫсяЇ | ЕкЖўВНЃКАБЛљМзЫсяЇЭбЫЎЩњГЩФђЫи |
2NH3(l)ЃЋCO2(g) | H2NCOONH4(l)
|
ПьЫйЗХШШ | Т§ЫйЮќШШ |
ЃЈ1ЃЉаДГіжЦБИФђЫиЕФзмЗДгІЛЏбЇЗНГЬЪНЃК____________________________ЃЌИУЗДгІШШЮЊ![]() ЃЌдђ
ЃЌдђ![]() ______________ЃЈЁАДѓгкЁБЁАаЁгкЁБЛђЁАЕШгкЁБЃЉ
______________ЃЈЁАДѓгкЁБЁАаЁгкЁБЛђЁАЕШгкЁБЃЉ![]() ЁЃ
ЁЃ
ЃЈ2ЃЉЯТСаЫЕЗЈе§ШЗЕФЪЧ________________ЁЃ
A.РћгУЖўбѕЛЏЬМжЦБИФђЫиЪЧМѕЛКЮТЪваЇгІЕФгааЇЗНЗЈ
B.ЕкЖўВНЗДгІИпЮТЬѕМўЯТздЗЂНјаа
C.ЬсИпЭЖСЯжаЕФЫЎЬМБШ гаРћгкФђЫиЕФЩњГЩ
гаРћгкФђЫиЕФЩњГЩ
D.ЕквЛВНЗДгІЕФЛюЛЏФмДѓгкЕкЖўВНЗДгІ
ЃЈ3ЃЉФђЫиЩњВњЙ§ГЬжазЊЛЏТЪЭЈГЃгУЖўбѕЛЏЬМзЊЛЏТЪРДБэЪОЃЌЕБЖўбѕЛЏЬМЦ№ЪМХЈЖШЮЊ![]() ЪБЃЈ
ЪБЃЈ![]() ЃЌЫЎЬМБШ=0.5ЃЉЃЌФђЫиЦНКтзЊЛЏТЪЫцАБЬМБШ
ЃЌЫЎЬМБШ=0.5ЃЉЃЌФђЫиЦНКтзЊЛЏТЪЫцАБЬМБШ ЕФБфЛЏШчБэЫљЪОЃК
ЕФБфЛЏШчБэЫљЪОЃК
АБЬМБШ/ХЈЖШБШ | 2.95 | 3.10 | 3.20 | 3.50 |
ФђЫиЦНКтзЊЛЏТЪ/% | 56.4 | 57.5 | 57.9 | 60.0 |
ЬсИпАБЬМБШгаРћгкЩњГЩФђЫиЃЌжївЊгаСНИідвђЃКвЛЪЧдіДѓАБЦјХЈЖШгаРћгкЗДгІе§ЯђвЦЖЏЃЛЖўЪЧ_____________ЁЃАБЬМБШЮЊ3.50ЪБЃЌЧѓИУзДЬЌЯТЕФжЦБИФђЫизмЗДгІЦНКтГЃЪ§K=___________.
ЃЈ4ЃЉвЛЖЈЬѕМўЯТЃЌдкЭМжаЛцжЦАБЛљМзЫсяЇЃЈ![]() ЃЉдкЗДгІЙ§ГЬжаЮяжЪЕФСПгыЪБМфЕФЙиЯЕЭМЁЃ
ЃЉдкЗДгІЙ§ГЬжаЮяжЪЕФСПгыЪБМфЕФЙиЯЕЭМЁЃ
 _______________
_______________
ЃЈ5ЃЉЭЈЙ§жБНгФђЫиШМСЯЕчГизАжУЃЌЪЕЯжСЫЁАФђЫиФмЁБЕФРћгУЃЌЧвВњЩњЮоЮлШОЕФВњЮяЃЌаДГіИКМЋЗДгІЃК____________________________.

ЁОД№АИЁП![]() Дѓгк AB Й§СПАБЦјгыЕкЖўВНЗДгІЩњГЩЕФЫЎНсКЯЃЌДйНјЦНКте§ЯђвЦЖЏ 0.13
Дѓгк AB Й§СПАБЦјгыЕкЖўВНЗДгІЩњГЩЕФЫЎНсКЯЃЌДйНјЦНКте§ЯђвЦЖЏ 0.13  ЃЈГіЯжЯШЩЯЩ§КѓЯТНЕдйЮШЖЈЧїЪЦМДЕУЗжЃЉ
ЃЈГіЯжЯШЩЯЩ§КѓЯТНЕдйЮШЖЈЧїЪЦМДЕУЗжЃЉ ![]()
ЁОНтЮіЁП
ЃЈ1ЃЉвРОнИЧЫЙЖЈТЩЗжЮіЃЛ
ЃЈ2ЃЉИљОнЗДгІздЗЂадЁЂЗДгІЫйТЪКЭЛЏбЇЦНКтЕФЛљБОдРэзїД№ЃЛ
ЃЈ3ЃЉДгХЈЖШгАЯьЛЏбЇЦНКтЕФНЧЖШПМТЧЃЛИљОнЬтвтЃЌСаГіШ§ЖЮЪНЃЌИљОнЦНКтГЃЪ§БэДяЪНСаЪНМЦЫуЃЛ
ЃЈ4ЃЉвРОнСНВНЗДгІЗжЮізїД№ЃЛ
ЃЈ5ЃЉгЩЬтФПаХЯЂПЩжЊдЕчГиИКМЋЗЂЩњФђЫиЕФбѕЛЏЗДгІЃЌдкМюадЬѕМўЯТНјааЃЌЙЪЩњГЩЬМЫсИљЁЃ
ЃЈ1ЃЉгЩЬтвтПЩжЊСНВНЗДгІЯрМгМДЮЊзмЗДгІЃЌЙЪжЦБИФђЫиЕФзмЗДгІЛЏбЇЗНГЬЪНЮЊЃК![]() ЃЛИљОнИЧЫЙЖЈТЩЃЌ
ЃЛИљОнИЧЫЙЖЈТЩЃЌ![]() ЃЌдђга
ЃЌдђга![]() ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК![]() ЃЛДѓгкЃЛ
ЃЛДѓгкЃЛ
ЃЈ2ЃЉA. гУгкЩњВњФђЫиЪЧЯћКФЖўбѕЛЏЬМЕФгааЇЭООЖЃЌAЯюе§ШЗЃЛ
B. ЕкЖўВНЗДгІ![]() ЃЌ
ЃЌ![]() ЃЌИпЮТздЗЂЃЌBЯюе§ШЗЃЛ
ЃЌИпЮТздЗЂЃЌBЯюе§ШЗЃЛ
C. ЫЎЪЧЕкЖўВНЗДгІВњЮяЃЌЬсИпЫЎЬМБШВЛРћгкЬсИпФђЫиЕФЩњГЩЃЌCЯюДэЮѓЃЛ
D. ЕквЛВНЗДгІПьЫйЃЌЕкЖўВНЗДгІТ§ЫйЃЌЙЪЕквЛВНЗДгІЛюЛЏФмаЁгкЕкЖўВНЗДгІЃЌDЯюДэЮѓЃЛ
ЙЪД№АИЮЊABЁЃ
ЃЈ3ЃЉЙ§СПАБЦјНсКЯЕкЖўВНЗДгІЩњГЩЕФЫЎЃЌДйНјЦНКте§ЯђвЦЖЏЃЛгЩЬтФПжаЕФЫЎЬМБШКЭАБЬМБШЃЌНсКЯФђЫиЦНКтзЊЛЏТЪЃЌМйЩшПЊЪМ![]() ЃЌ
ЃЌ
![]()
ПЊЪМ![]() 7 2 0
7 2 0
зЊЛЏ![]() 2.4 1.2 1.2
2.4 1.2 1.2
ЦНКт![]() 4.6 0.8 1.2ЃЌ
4.6 0.8 1.2ЃЌ
вђЫЎЬМБШЮЊ0.5ЃЌЦ№ЪМЫЎеєЦјЕФХЈЖШЮЊ2mol/L![]() 0.5=1mol/LЃЌЙЪЫЎеєЦјЕФЦНКтХЈЖШЮЊ1.2mol/L+1mol/L=2.2mol/LЃЌдђЦНКтГЃЪ§
0.5=1mol/LЃЌЙЪЫЎеєЦјЕФЦНКтХЈЖШЮЊ1.2mol/L+1mol/L=2.2mol/LЃЌдђЦНКтГЃЪ§![]() ЃЛ
ЃЛ
ЃЈ4ЃЉЕквЛВНЗДгІПьЫйЩњГЩАБЛљМзЫсяЇЃЌЕкЖўВНЗДгІЯћКФНЯТ§ЃЌЙЪАБЛљМзЫсяЇЯШРлЛ§КѓДяЕНЦНКтХЈЖШЃЌЙЪД№АИЮЊЃК ЃЈГіЯжЯШЩЯЩ§КѓЯТНЕдйЮШЖЈЧїЪЦМДЕУЗжЃЉЃЛ
ЃЈГіЯжЯШЩЯЩ§КѓЯТНЕдйЮШЖЈЧїЪЦМДЕУЗжЃЉЃЛ
ЃЈ5ЃЉНсКЯИКМЋФђЫиВњЩњЕЊЦјКЭЬМЫсИљРызгЃЌЦфЕчМЋЗДгІЪНЮЊЃК![]() ЁЃ
ЁЃ

ЁОЬтФПЁПУОКЯН№МАУОЕФЛЏКЯЮядкЩњВњЁЂЩњЛюжагазХЙуЗКЕФгІгУЁЃ
(1)УОдкдЊЫижмЦкБэжаЕФЮЛжУЪЧ__________________ЁЃ
(2)гУЫЎТШУОЪЏ(жївЊГЩЗжЮЊMgCl2ЁЄ6H2O)жЦБИН№ЪєУОЕФЙиМќСїГЬШчЯТЃК

ИУЙЄвежаПЩбЛЗЪЙгУЕФЮяжЪга______________ЁЃ
(3)ДЂЧтВФСЯMg(AlH4)2дк110ЁЋ200 ЁцЕФЗДгІЮЊMg(AlH4)2=MgH2ЃЋ2AlЃЋ3H2ЁќЃЌУПзЊвЦ6 molЕчзгЩњГЩЧтЦјЕФЮяжЪЕФСПЮЊ________molЁЃ
(4)МюЪНЬМЫсУОУмЖШаЁЃЌЪЧЯ№НКжЦЦЗЕФгХСМЬюСЯЃЌПЩгУИДбЮMgCO3ЁЄ(NH4)2CO3ЁЄ2H2OзїдСЯжЦБИЁЃжЦБИЙ§ГЬжаЃЌашвЊгУЕНТБЫЎ(ТШЛЏУОШмвК)ЁЃФГПЦбааЁзщгУГСЕэЕЮЖЈЗЈЗжЮіВњЦЗжаClЃЕФКЌСПЃЌГЦШЁ6.100 0 gВњЦЗгУЪЪСПЯѕЫсШмНтЃЌОЯЁЪЭЕШВНжшзюжеХфЕУ500 mLЕФШмвКЁЃ
aЃЎзМШЗСПШЁ25.00 mL Д§ВтвКЃЌгУ0.100 0 mol/L AgNO3БъзМвКЕЮЖЈЃЌЕЮЖЈЧАКѓЕЮЖЈЙмжаЕФвКУцЖСЪ§ШчЭМЫљЪОЃЌдђЕЮЖЈЙ§ГЬжаЯћКФБъзМвКЕФЬхЛ§ЮЊ________mLЁЃ

b.
AgCl | AgBr | AgI | Ag2CrO4 | |
Ksp | 2ЁС10Ѓ10 | 5.4ЁС10Ѓ13 | 8.3ЁС10Ѓ17 | 2ЁС10Ѓ12 |
беЩЋ | Аз | ЕЛЦ | ЛЦ | зЉКь |
ВЮееЩЯБэЪ§ОнМАаХЯЂЗжЮіЃЌЕЮЖЈЪБПЩвдзїжИЪОМСЕФЪЧ________(ЬюЪ§зжађКХ)ЁЃ
ЂйCaCl2ЁЁЁЁЂкNaBrЁЁЁЁЂлNaIЁЁЁЁЂмK2CrO4
ЁОЬтФПЁПЖўбѕЛЏЬМРћгУОпгаЪЎЗжживЊЕФвтвхЃЌПЦбЇМвгавдЯТМИИіЩшЯыЁЃ
(1)гУЬЋбєФмНЋCO2зЊЛЏГЩO2КЭC(ЪЏФЋЯЉЃЉЃЌЦфЩшЯыШчЭМЃК

дђжиећЯЕЭГЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_________ЁЃ

(2)ЖўбѕЛЏЬМКЭЧтЦјдкДпЛЏМСзїгУЯТПЩжЦШЁЕЭЬМЯЉЬўЁЃдквЛУмБеШнЦїжаЗжБ№ЭЖШы1molCO2ЁЂ3molH2ЃЌЗЂЩњЗДгІЃК2CO2(g)+6H2(g)![]() C2H4(g)+ 4H2O(g) ЁїHЃЛдкВЛЭЌЮТЖШЯТЃЌгУДЋИаММЪѕВтГіЦНКтЪБH2ЕФЮяжЪЕФСПБфЛЏЙиЯЕШчЭМЫљЪОЁЃ
C2H4(g)+ 4H2O(g) ЁїHЃЛдкВЛЭЌЮТЖШЯТЃЌгУДЋИаММЪѕВтГіЦНКтЪБH2ЕФЮяжЪЕФСПБфЛЏЙиЯЕШчЭМЫљЪОЁЃ
ЂйЦфЫќЬѕМўВЛБфЃЌЦ№ЪМЪБШєАДlmolCO2ЁЂ2molH2НјааЭЖСЯЃЌCO2зЊЛЏТЪНЋ________(ЬюЁАдіДѓЁБЁЂЁА МѕаЁЁБЛђЁАВЛБфЁБЃЉЃЛ
ЂкЁїH_____0ЃЈЬюЁА>ЁБЁА<ЁБЁА ВЛФмШЗЖЈЁБЃЉЁЃ
ЂлШєВтЪджаЬхЯЕФкЮобѕЦјВњЩњЃЌЪдНсКЯЭМЪОЭЦЖЯШШЮШЖЈадC2H4 _____H2O ЃЈЬюЁА>ЁБЁА<ЁБЁА ВЛФмШЗЖЈЁБЃЉЁЃ
(3)гУАБЫЎЮќЪеCO2жЦЛЏЗЪ(NH4HCO3)
ЂйвбжЊЃКNH3ЁЄH2O(aq)![]() NH4+(aq)+OH-(aq) ЁїH1=akJ/mol
NH4+(aq)+OH-(aq) ЁїH1=akJ/mol
CO2(g)+H2O(l)![]() H2CO3(aq) ЁїH2=bkJ/mol
H2CO3(aq) ЁїH2=bkJ/mol
H2CO3(aq)+OH-(aq)![]() HCO3-(aq)+H2O(l) ЁїH3=ckJ/mol
HCO3-(aq)+H2O(l) ЁїH3=ckJ/mol
дђРћгУNH3 H2OЮќЪеCO2жЦБИNH4HCO3ЕФШШЛЏбЇЗНГЬЪНЮЊ______________________ЃЛ
ЂквбжЊГЃЮТЯТЯрЙиЪ§ОнШчБэЃК
Kb(NH3ЁЄH2O) | 2ЁС10-5kJ/mol |
Ka1(H2CO3) | 4ЁС10-7kJ/mol |
Ka2(H2CO3) | 4ЁС10-11kJ/mol |
дђЗДгІNH4++HCO3-+H2O![]() NH3 H2O+H2CO3ЕФЦНКтГЃЪ§K=___________ЁЃ
NH3 H2O+H2CO3ЕФЦНКтГЃЪ§K=___________ЁЃ
ЁОЬтФПЁПЕўЕЊЛЏМиЃЈ![]() ЃЉФмДйЪЙзїЮяЛђФбгкУШЗЂЕФжжзгЗЂг§ЁЃЩшМЦШчЯТЪЕбщжЦБИЕўЕЊЛЏМиВЂВтЖЈЦфДПЖШЃК
ЃЉФмДйЪЙзїЮяЛђФбгкУШЗЂЕФжжзгЗЂг§ЁЃЩшМЦШчЯТЪЕбщжЦБИЕўЕЊЛЏМиВЂВтЖЈЦфДПЖШЃК
I.жЦБИ
ВНжш1ЃКжЦБИбЧЯѕЫсЖЁѕЅЃЈ![]() ЃЉ
ЃЉ
![]()
ЗДгІзАжУШчЭМ1ЃЈМаГжзАжУТдШЅЃЉЃЌЯђЩеБжавРДЮМгШыЯЁСђЫсЁЂЖЁДМЁЂбЧЯѕЫсФЦШмвКЃЌД§ЗДгІЭъШЋКѓЃЌЗжРыГіЩЯВугЭзДЮяЃЌгУ![]() КЭ
КЭ![]() ЕФЛьКЯШмвКЯДЕгШ§ДЮЃЌОИЩдяКѓБИгУЁЃ
ЕФЛьКЯШмвКЯДЕгШ§ДЮЃЌОИЩдяКѓБИгУЁЃ
ВНжш2ЃКжЦБИЕўЕЊЛЏМи
![]()
ЗДгІзАжУШчЭМ2ЃЈМаГжМАМгШШзАжУТЗШЅЃЉЃЌЯђвЧЦїAжаМгШы![]() ввДМШмвКЁЂ
ввДМШмвКЁЂ![]() ЕФСЊАБЃЈ
ЕФСЊАБЃЈ![]() ЃЉЁЂбЧЯѕЫсЖЁѕЅЃЌеєЦћдЁМгШШЃЌЗДгІЭъШЋКѓЃЌЕўЕЊЛЏМиМДГСЕэГіРДЃЌБљдЁРфШДЃЌЙ§ТЫЃЌЯШгУЮоЫЎввДМЯДЕгЃЌдйгУЮоЫЎввУбЯДЕгЃЌдкПеЦјжагк
ЃЉЁЂбЧЯѕЫсЖЁѕЅЃЌеєЦћдЁМгШШЃЌЗДгІЭъШЋКѓЃЌЕўЕЊЛЏМиМДГСЕэГіРДЃЌБљдЁРфШДЃЌЙ§ТЫЃЌЯШгУЮоЫЎввДМЯДЕгЃЌдйгУЮоЫЎввУбЯДЕгЃЌдкПеЦјжагк![]() ИЩдяЁЃ
ИЩдяЁЃ


ЯрЙиЮяжЪаджЪШчЯТЃК
ЮяжЪ | беЩЋЁЂзДЬЌ | ЗаЕуЃЈЁцЃЉ | ШмНтад |
| ЮоЩЋОЇЬх | ЪмШШвзЗжНт | взШмгкЫЎЃЌЮЂШмгкввДМЃЌВЛШмгкввУб |
| ЮоЩЋвКан | 118 | ЮЂШмгкЫЎЃЌгыввДМЁЂввУбЛьШм |
| ЮоЩЋЛђЕЛЦЩЋгЭзДвКЬх | 78 | ВЛШмгкЫЎЃЌгыввДМЁЂввУбЛьШм |
| ЮоЩЋгЭзДвКЬх | 118 | гыЫЎЁЂввДМЛьШмЃЌВЛШмгкввУб |
ЧыЛиД№ЃК
ЃЈ1ЃЉвЧЦїAЕФУћГЦЮЊ_____________.
ЃЈ2ЃЉВНжш1жаЗжРыГібЧЯѕЫсЖЁѕЅЕФВйзїУћГЦЮЊ_____________ЃЛВНжш1жагУNaClКЭNaHCO3ЕФЛьКЯШмвКЯДЕгЕФФПЕФЪЧ__________________________.
ЃЈ3ЃЉВНжш2жаБљдЁРфШДЕФФПЕФЪЧ__________________________ЃЛВНжш2жаИЩдяВњЦЗЕФЮТЖШПижЦдк55~60ЁцЃЌдвђЪЧ__________________________
ЃЈ4ЃЉШчашЬсИпВњЦЗЕФДПЖШЃЌПЩдк_____________ЃЈЬюБрКХЃЉжаНјаажиНсОЇЁЃ
A.ЮоЫЎввДМ B.ЮоЫЎввУб C.ЫЎ D.ввДМЕФЫЎШмвК
Ђђ.ЗжЙтЙтЖШЗЈВтЖЈВњЦЗЕФДПЖШ
дРэЃК![]() гы
гы![]() ЗДгІЗЧГЃСщУєЃЌЩњГЩКьЩЋТчКЯЮяЃЌдквЛЖЈВЈГЄЯТВтСПКьЩЋШмвКЕФЮќЙтЖШЃЌРћгУЁА
ЗДгІЗЧГЃСщУєЃЌЩњГЩКьЩЋТчКЯЮяЃЌдквЛЖЈВЈГЄЯТВтСПКьЩЋШмвКЕФЮќЙтЖШЃЌРћгУЁА![]() ЮќЙтЖШЁБЧњЯпШЗЖЈбљЦЗШмвКжаЕФ
ЮќЙтЖШЁБЧњЯпШЗЖЈбљЦЗШмвКжаЕФ![]() ЁЃВтЖЈВНжшШчЯТЃК
ЁЃВтЖЈВНжшШчЯТЃК
ЂйгУ![]() ЦЗЬхХфжЦ
ЦЗЬхХфжЦ![]() БъзМШмвКЃЛ
БъзМШмвКЃЛ
ЂкХфжЦвЛзщЯрЭЌЬхЛ§ЃЈ![]() ЃЉВЛЭЌХЈЖШЕФ
ЃЉВЛЭЌХЈЖШЕФ![]() БъзМШмвКЃЌЗжБ№МгШы
БъзМШмвКЃЌЗжБ№МгШы![]() ЃЈзуСПЃЉ
ЃЈзуСПЃЉ![]() БъзМШмвКЃЌвЁдШЃЌВтСПЮќЙтЖШЃЌЛцжЦБъзМШмвКЕФ
БъзМШмвКЃЌвЁдШЃЌВтСПЮќЙтЖШЃЌЛцжЦБъзМШмвКЕФ![]() гыЮќЙтЖШЕФЙиЯЕЧњЯпЃЌШчЭМЃЛ
гыЮќЙтЖШЕФЙиЯЕЧњЯпЃЌШчЭМЃЛ
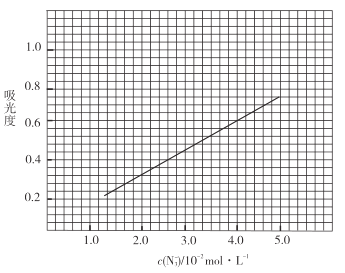
ЂлВњЦЗВтЖЈЃКГЦШЁ0.360gВњЦЗЃЌХфГЩ![]() ШмвКЃЌШЁГі
ШмвКЃЌШЁГі![]() гкБъзМЙмжаЃЌМгШы
гкБъзМЙмжаЃЌМгШы![]() ЃЈзуСПЃЉ
ЃЈзуСПЃЉ![]() БъзМШмвКЃЌвЁдШЃЌВтЕУЮќЙтЖШЮЊ0.6ЁЃ
БъзМШмвКЃЌвЁдШЃЌВтЕУЮќЙтЖШЮЊ0.6ЁЃ
ЃЈ5ЃЉЪЕбщЪвгУ![]() ОЇЬхХфжЦ
ОЇЬхХфжЦ![]() БъзМШмвКЕФЗНЗЈЮЊ_________________.
БъзМШмвКЕФЗНЗЈЮЊ_________________.
ЃЈ6ЃЉВњЦЗЕФДПЖШЮЊ_________________ЃЛШєЂлжаМгШыЕФ![]() БъзМШмвКВЛзувдНЋВњЦЗЭъШЋЗДгІЃЌдђВтЕУЕФВњЦЗДПЖШ________________ЃЈЬюЁАЦЋИпЁБЁАЦЋЕЭЁБЛђЁАЮогАЯьЁБЃЉЁЃ
БъзМШмвКВЛзувдНЋВњЦЗЭъШЋЗДгІЃЌдђВтЕУЕФВњЦЗДПЖШ________________ЃЈЬюЁАЦЋИпЁБЁАЦЋЕЭЁБЛђЁАЮогАЯьЁБЃЉЁЃ




