��Ŀ����
��֪���������������������壩��H2�ڸ�����������ʱҪ�Է�����Ӧ�����������ͽ����⻯���
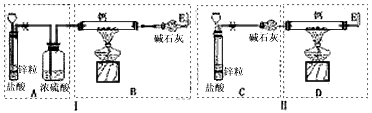
NaH��CaH2�ȣ���������ˮ��Ӧ�����мס�����λͬѧ�ֱ�������Ʊ�CaH2��ʵ�飬װ����ͼ��ʾ���ֱ�����Ţ��ʾ��������̨�ȼг̶ֹ���������ȥ��
NaH��CaH2�ȣ���������ˮ��Ӧ�����мס�����λͬѧ�ֱ�������Ʊ�CaH2��ʵ�飬װ����ͼ��ʾ���ֱ�����Ţ��ʾ��������̨�ȼг̶ֹ���������ȥ��
��ش���������
��1��п�����ᷴӦ�����ӷ���ʽΪ__________________________��
��2�����ʵ������ʾ����λͬѧ��ʵ��װ����ƶ���ȱ�ݡ�װ�â�IJ���֮����________________��װ�â�IJ���֮����__________________________��
��3�����������װ����ѡȡ����Ϊ�����IJ��֣��������ҵ�˳����װһ����ȡCaH2�ĺ���װ�ã������A��B��C������ʾ��_______________________��
��4�������װ�õ������Ժ�Ϊ�˱�֤��Ʒ�Ĵ��Ⱥ�ʵ�鰲ȫ������_________________��Ȼ����____________________�����ܵ�ȼ�ƾ��Ƽ��ȡ�ͬ����Ϊ�˰�ȫ����Ӧ��ʼ����E�ڴ�Ӧ_______________��
��5�����º�����Ұ����ҵ����CaH2���������ʹ֮��ˮ��Ӧ����H2���䷴Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��1��п�����ᷴӦ�����ӷ���ʽΪ__________________________��
��2�����ʵ������ʾ����λͬѧ��ʵ��װ����ƶ���ȱ�ݡ�װ�â�IJ���֮����________________��װ�â�IJ���֮����__________________________��
��3�����������װ����ѡȡ����Ϊ�����IJ��֣��������ҵ�˳����װһ����ȡCaH2�ĺ���װ�ã������A��B��C������ʾ��_______________________��
��4�������װ�õ������Ժ�Ϊ�˱�֤��Ʒ�Ĵ��Ⱥ�ʵ�鰲ȫ������_________________��Ȼ����____________________�����ܵ�ȼ�ƾ��Ƽ��ȡ�ͬ����Ϊ�˰�ȫ����Ӧ��ʼ����E�ڴ�Ӧ_______________��
��5�����º�����Ұ����ҵ����CaH2���������ʹ֮��ˮ��Ӧ����H2���䷴Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��1��Zn+2H+=Zn2++H2��
��2����H2װ�ú�ȱ�ٳ�HCl�����װ�ã���CaH2װ�ú�ȱ�ٸ���װ��
��3��C��B
��4��ͨ����������������������ꣻ��ȼβ�������������ȣ�
��5��CaH2+2H2O=Ca(OH)2+2H2��
��2����H2װ�ú�ȱ�ٳ�HCl�����װ�ã���CaH2װ�ú�ȱ�ٸ���װ��
��3��C��B
��4��ͨ����������������������ꣻ��ȼβ�������������ȣ�
��5��CaH2+2H2O=Ca(OH)2+2H2��

��ϰ��ϵ�д�
 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�
�����Ŀ


