��Ŀ����
(10��) (1)��һ�������£��ݻ�Ϊ 10 L�ܱ������з�����Ӧ��
CH4(g)+H2O(g)
 CO(g)+3H2(g)����H��0
CO(g)+3H2(g)����H��0
��1.0 mol CH4��2.0 mol H2O(g)ͨ����ܱ����� 3 sʱ��0.1 mol CO���ɣ���3 s�ڸ÷�Ӧ��ƽ������v(H2)= ��
(2)��ѹǿΪ0.1
MPa������,�ݻ�ΪV Lij�ܱ�������a
mol CO�� 2a mol H2�ڴ��������·�Ӧ���ɼ״���CO(g)+2H2(g)  CH3OH(g)��CO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
CH3OH(g)��CO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
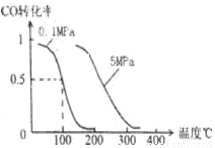
�ٸ÷�Ӧ�� ��Ӧ(����ȡ������ȡ�)��
��150��ʱ�÷�Ӧ��ƽ�ⳣ��K V2��a2(���������������)��
�����¶��ݻ����������£�����ܱ�����������a mol CO�� 2a mol H2����b mol CH3OH(g)����ﵽ��ƽ��ʱ��CO��ת���� (���������С�����䡱����ȷ����)��ƽ�ⳣ�� (���������С�����䡱)��
���𰸡�

��������

��ϰ��ϵ�д�
�����Ŀ



 Mg2����2OH��������ϵ�м��루��������ֲ�ͬ�������ʣ�_____________________________________________________ ������Mg(OH)2�ܽ⡣
Mg2����2OH��������ϵ�м��루��������ֲ�ͬ�������ʣ�_____________________________________________________ ������Mg(OH)2�ܽ⡣