��Ŀ����
2012��3��21 ���ǵڶ�ʮ�조����ˮ�ա�������ˮ��Դ����������
(1)ClO2��Cl2(��ԭ���ﶼΪCl��)�������г��õ��������������ĵ����ʵ�������������ʱ��ClO2������Ч����Cl2��________����
(2)ij��ɫ��ˮ�п��ܺ���Fe3����Al3����Mg2����Na����NO3����CO32����SO42�������еļ��֣�Ϊ������ɷ֣��ֱ�ȡ��ˮ��Ʒ100 mL������������ʵ�飬��������й���������ͼ��ʾ��

�������ͼ�ش��������⣺
��ʵ����������1.0 mol/L��NaOH��Һ100 mL�������������˲�������������ƽ����Ͳ��ҩ�ס��ձ�����ͷ�ιܣ���ȱ�ٵ�����Ϊ_________________________________________��
��ʵ����г�������A��B��������������Ӧ�����ӷ���ʽΪ____________________��
����ȷ��NO3���Ƿ���ڣ�________(����ڡ����������ڡ���ȷ����)�������ڣ��Լ���c(NO3��)��________(�������ڣ����ʲ�������)��
(1)2.5��(2)��100mL����ƿ����Al(OH)3��OH��=AlO2����2H2O���۴��ڡ�0.15 mol/L
����

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�ͭ����Ͻ�����������ʹ�õĽ������ϡ�
(1)����ͭ��ȡ�������ַ�ʽ�ѻ�(����)

(2)��1��Cu2O������(�ṹ����ͼ��ʾ)��Cuԭ����λ��Ϊ__________��
(3)��ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�
�ٵ����Ļ�ѧʽ����������ʽ��ʾΪ____________��
�ڵ�����SO42���Ŀռ乹��Ϊ________��H2O��Oԭ�ӵ��ӻ�����Ϊ________��
��ij��ȤС���ȡ2.500 g�������壬������ʹ��ʧˮ����ȷ�ⶨ��ͬ�¶���ʣ�������������õ���ͼ��ʾ��ʵ����ʾ��ͼ������˵����ȷ����(����)
| A������ӳ�������105 ��Ĺ�����ֻ��������� |
| B�������������γ���λ����4��ˮ����ͬʱʧȥ |
| C��120 ��ʱ��ʣ�����Ļ�ѧʽ��CuSO4��H2O |
| D������������ʧˮʱ���˷�����������С��ͬ�������е�ˮ���ӿ��Է�Ϊ3�� |
��.ʵ��������1mol/L Na2CO3��Һ250ml��
��1����Ҫ����Na2CO3 g����2������Һ�е���������ĿΪ ����
��3����Ҫ���ʵ���Ũ��Ϊ5mol/L ��Na2CO3��Һ ml��
��4��������Һ������ϡ���ᷴӦ�������������ڱ�״���µ����Ϊ L��
��5�����Ƹ���Һ�IJ���˳����(����ĸ��ʾ,���ظ�ʹ��) ��
| A������ | B��ϴ�� | C������ | D���ܽ� E��ҡ�� F��ת�� |
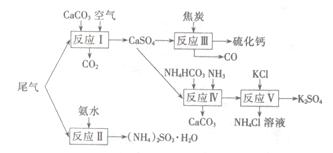
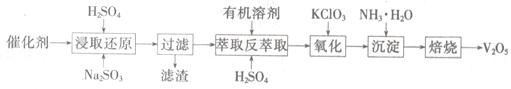
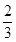 ʱ��b�� ���õ���CO��CO2�����ʵ���֮��n��CO����n��CO2���� ��
ʱ��b�� ���õ���CO��CO2�����ʵ���֮��n��CO����n��CO2���� ��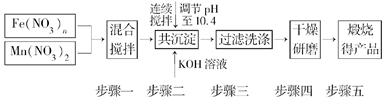
 MnFe2O4��x��O2����
MnFe2O4��x��O2���� MnFe2O4��xH2��
MnFe2O4��xH2��