��Ŀ����
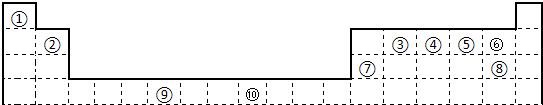
�±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�| �� | |||||||||||||||||
| �� | �� | �� | �� | �� | |||||||||||||
| �� | �� | ||||||||||||||||
| �� | �� |
��2����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ��______�ӻ���Ԫ�آ�����γɵĻ�����ľ��������ǣ�______��
��3��Ԫ�آܵĵ�һ������______Ԫ�آݣ�ѡ���������=�������������ĵ�һ�����ܣ�Ԫ�آ���Ԫ�آ��γɵ�X���ӵĿռ乹��Ϊ��______����д����N2��Ϊ�ȵ�����ķ��ӡ����ӵĻ�ѧʽ______��______����дһ�֣���
��4���ڲⶨ������γɻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ���ǣ�______��
��5��Ԫ�آܵ�����������Ӧ��ˮ����ϡ��Һ��Ԫ�آߵĵ��ʷ�Ӧʱ��Ԫ�آܱ���ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ�ǣ�______��
��6����������Xͨ�뺬��Ԫ�آ����ɫ��������Һ�У������ӷ���ʽΪ��______��
���𰸡�����������Ԫ�����ڱ��ṹ������ȷ���١��ڡ��ۡ��ܡ��ݡ��ޡ��ߡ��ࡢ�ᡢ��Ԫ�طֱ�Ϊ��H��Be��C��N��O��F��Mg��Cl��
Cr��Cu��
��1������CrԪ�أ�ԭ������Ϊ24�����ݺ�������Ų�������д��
��2������CԪ�أ�����HԪ�أ��γɵ�ˮ����������廯��������ϩ���ӻ�����������ɹ¶Ե��ӶԺͳɦҼ���
����MgԪ�أ�����ClԪ�أ����ý�������÷ǽ����γ����ӻ����
��3������NԪ�أ�����OԪ�أ�ͬ����Ԫ�ص�һ�����ܴ���������������ƣ�������ͬ��ԭ�ӹ����ȫ����������ȫ��ʱ��ϵ������ͣ�ԭ�ӽ��ȶ�����˼۵����Ų����ڰ����Ĺ����Ԫ�أ����һ�����ܱ��ٽ�ԭ�ӵĵ�һ�����ܴ�
Ԫ�آ���Ԫ�آ��γɵ���NH3���ӣ�Ϊ�����Σ�
ԭ����Ŀ�͵�����������۵�����������ͬ������Ϊ�ȵ����壬�ȵ�����������ƵĽṹ������
��4��F�ĵ縺�Դ�HF���Ӽ���γ������
��5������NԪ�أ�����������Ӧ��ˮ����ϡ��ҺΪϡ���ᣬ��Mg���ʷ�Ӧ��Mg�ǻ�ԭ����������ΪMg��NO3��2��ϡ��������������������ã������������������ñ���ԭΪNH4NO3��
��6����������������ͭ��Һ��Ӧ����������ɫ�İ���ͭ�����ӣ�
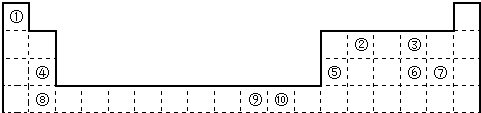
����⣺����Ԫ�����ڱ��ṹ������ȷ���١��ڡ��ۡ��ܡ��ݡ��ޡ��ߡ��ࡢ�ᡢ��Ԫ�طֱ�Ϊ��H��Be��C��N��O��F��Mg��
Cl��Cr��Cu��
��1������CrԪ�أ�Cr��24��Ԫ�أ���ԭ�Ӻ�����24�����ӣ������������ԭ�������ع�����д���������Ų�ʽ��3d�ܼ�����������4s�ܼ�����������������4s����3d������еĵ��Ӵ���ȫ����������ȫ��ʱԭ�����ȶ�������Cr�ĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1����Χ�����Ų�ʽ3d54s1��
�ʴ�Ϊ��3d54s1��
��2����ϩ����˫���͵���������̼̼˫����һ���ǦҼ���1��p-p�Ħм��������4��̼�����Ϊ�Ҽ���̼ԭ���ӻ������Ϊ3�����Բ���sp2�ӻ���ʽ��
���ý�������÷ǽ����γ����ӻ����Ԫ�آ�����γɵĻ��������Ȼ�þ���������ӻ�����γ����Ӿ��壮
�ʴ�Ϊ��sp2�����Ӿ��壻
��3��������NԪ�أ���Χ�����Ų�ʽ2s22p3������OԪ�أ���Χ�����Ų�ʽ2s22p4����Ԫ�ش���ͬһ���ڣ���ԭ��ԭ��������NԪ��P�ܼ����ڰ������������ͣ�ԭ�ӽ��ȶ������һ��������ԭ�ӵĵ�һ�����ܴ�
Ԫ�آ���Ԫ�آ��γɵ���NH3���ӣ�Nԭ�Ӳ�ȡsp3�ӻ�����һ�Թ¶Ե��Ӷԣ�����Ϊ�����Σ�
N2��2��ԭ�ӣ�14�����ӣ���10���۵��ӣ�����ȵ�����ΪCO��C22- �ȣ�
�ʴ�Ϊ�����������Σ�CO��C22-��
��4��F�ĵ縺�Դ�HF���Ӽ���γ������ʵ���õ�ֵһ���������ֵ��
�ʴ�Ϊ��HF���Ӽ���γ������
��5��Mg��ϡ���ᷴӦ����ʽΪ4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
��6����������������ͭ��Һ��Ӧ����������ɫ�İ���ͭ�����ӣ����ӷ���ʽΪCu2++4NH3=[Cu��NH3��4]2+��
�ʴ�Ϊ��Cu2++4NH3=[Cu��NH3��4]2+��
������������һ���ṹ��ѧ֪ʶ����Ŀ���ۺ��Խ�ǿ���ѶȽϴ�ע�����еĵ��Ӵ���ȫ����������ȫ��ʱԭ�����ȶ���
Cr��Cu��
��1������CrԪ�أ�ԭ������Ϊ24�����ݺ�������Ų�������д��
��2������CԪ�أ�����HԪ�أ��γɵ�ˮ����������廯��������ϩ���ӻ�����������ɹ¶Ե��ӶԺͳɦҼ���
����MgԪ�أ�����ClԪ�أ����ý�������÷ǽ����γ����ӻ����
��3������NԪ�أ�����OԪ�أ�ͬ����Ԫ�ص�һ�����ܴ���������������ƣ�������ͬ��ԭ�ӹ����ȫ����������ȫ��ʱ��ϵ������ͣ�ԭ�ӽ��ȶ�����˼۵����Ų����ڰ����Ĺ����Ԫ�أ����һ�����ܱ��ٽ�ԭ�ӵĵ�һ�����ܴ�
Ԫ�آ���Ԫ�آ��γɵ���NH3���ӣ�Ϊ�����Σ�
ԭ����Ŀ�͵�����������۵�����������ͬ������Ϊ�ȵ����壬�ȵ�����������ƵĽṹ������
��4��F�ĵ縺�Դ�HF���Ӽ���γ������
��5������NԪ�أ�����������Ӧ��ˮ����ϡ��ҺΪϡ���ᣬ��Mg���ʷ�Ӧ��Mg�ǻ�ԭ����������ΪMg��NO3��2��ϡ��������������������ã������������������ñ���ԭΪNH4NO3��
��6����������������ͭ��Һ��Ӧ����������ɫ�İ���ͭ�����ӣ�
����⣺����Ԫ�����ڱ��ṹ������ȷ���١��ڡ��ۡ��ܡ��ݡ��ޡ��ߡ��ࡢ�ᡢ��Ԫ�طֱ�Ϊ��H��Be��C��N��O��F��Mg��
Cl��Cr��Cu��
��1������CrԪ�أ�Cr��24��Ԫ�أ���ԭ�Ӻ�����24�����ӣ������������ԭ�������ع�����д���������Ų�ʽ��3d�ܼ�����������4s�ܼ�����������������4s����3d������еĵ��Ӵ���ȫ����������ȫ��ʱԭ�����ȶ�������Cr�ĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1����Χ�����Ų�ʽ3d54s1��
�ʴ�Ϊ��3d54s1��
��2����ϩ����˫���͵���������̼̼˫����һ���ǦҼ���1��p-p�Ħм��������4��̼�����Ϊ�Ҽ���̼ԭ���ӻ������Ϊ3�����Բ���sp2�ӻ���ʽ��
���ý�������÷ǽ����γ����ӻ����Ԫ�آ�����γɵĻ��������Ȼ�þ���������ӻ�����γ����Ӿ��壮
�ʴ�Ϊ��sp2�����Ӿ��壻
��3��������NԪ�أ���Χ�����Ų�ʽ2s22p3������OԪ�أ���Χ�����Ų�ʽ2s22p4����Ԫ�ش���ͬһ���ڣ���ԭ��ԭ��������NԪ��P�ܼ����ڰ������������ͣ�ԭ�ӽ��ȶ������һ��������ԭ�ӵĵ�һ�����ܴ�
Ԫ�آ���Ԫ�آ��γɵ���NH3���ӣ�Nԭ�Ӳ�ȡsp3�ӻ�����һ�Թ¶Ե��Ӷԣ�����Ϊ�����Σ�
N2��2��ԭ�ӣ�14�����ӣ���10���۵��ӣ�����ȵ�����ΪCO��C22- �ȣ�
�ʴ�Ϊ�����������Σ�CO��C22-��
��4��F�ĵ縺�Դ�HF���Ӽ���γ������ʵ���õ�ֵһ���������ֵ��
�ʴ�Ϊ��HF���Ӽ���γ������
��5��Mg��ϡ���ᷴӦ����ʽΪ4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
��6����������������ͭ��Һ��Ӧ����������ɫ�İ���ͭ�����ӣ����ӷ���ʽΪCu2++4NH3=[Cu��NH3��4]2+��
�ʴ�Ϊ��Cu2++4NH3=[Cu��NH3��4]2+��
������������һ���ṹ��ѧ֪ʶ����Ŀ���ۺ��Խ�ǿ���ѶȽϴ�ע�����еĵ��Ӵ���ȫ����������ȫ��ʱԭ�����ȶ���

��ϰ��ϵ�д�
 ��������ѧ����ϵ�д�
��������ѧ����ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
�����Ŀ