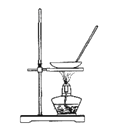
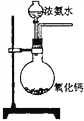
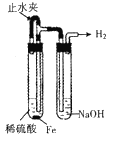
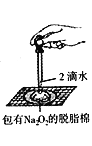
��Ŀ����
����Ŀ��25��ʱ�� c mol��L��1CH3COOH ��Һ��ˮϡ�ͣ� ��Һ�� CH3COOH �� CH3COO�������и�����ռ�����ʵ�������(��)����Һ pH �仯�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����
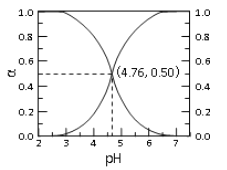
A. ��ͼ��֪�� 25��ʱ����� Ka=10-4.76
B. c mol��L��1CH3COOH ��Һ��ˮϡ���У� ��(CH3COOH)���� c(CH3COO��)Ҳһ������
C. �� pH��4.76 ����Һ��ͨ�� HCl�� ��(CH3COOH)������(CH3COO��)��С�� ��(CH3COOH)+��(CH3COO��)=1
D. ����ͼ��������������һ������Ӧ����Һ�У����� c(CH3COO��)+c(OH��)=c(H+)
���𰸡�B
��������A. ��ͼ��֪��pH=4.76ʱ����(CH3COOH)=c(CH3COO��) ������25��ʱ����� Ka=10-4.76��A��ȷ��B. c mol��L��1CH3COOH ��Һ��ˮϡ���У�CH3COOH�ĵ���ƽ�������ƶ���������(CH3COOH)��С�� c(CH3COO��)������B����ȷ��C. �� pH��4.76 ����Һ��ͨ�� HCl��HCl����ʹ��Һ��c(H+)����CH3COOH�ĵ���ƽ�������ƶ� ��������(CH3COOH)������(CH3COO��)��С�����������غ��֪�� ��(CH3COOH)+��(CH3COO��)=1��C��ȷ��D. ���������غ��֪������ͼ��������������һ������Ӧ����Һ�У����� c(CH3COO��)+c(OH��)=c(H+)��D��ȷ������ѡB.

����Ŀ���п�ѧ�������о���̼��һ��������Ӧ������������
C��s��+2NO��g��![]() CO2��g��+N2��g����H
CO2��g��+N2��g����H
(1)ʵ�鷽���������д�ʩ�������ü�����߷�Ӧ��������ʹ�ô�����߷�Ӧ��������ʹ�ü�ѹ���NOת��������ʹCO2ת���ɸɱ�����ϵ�����룬���NO��ת����������Ϊ���е���_________��
(2)�����Ӧ��ƽ�ⳣ������ʽ��_________________��
(3)�ں��ݺ����ܱ������У���ѧ�ҵõ�����ʵ������
ʱ�䣨min�� | Ũ�ȣ�mol/L�� | ||
NO | N2 | CO2 | |
0 | 0.100 | 0 | 0 |
10 | 0.058 | 0.021 | 0.021 |
20 | 0.040 | 0.030 | 0.030 |
30 | 0.040 | 0.030 | 0.030 |
��Ӧ�ڸ��¶��µ�ƽ�ⳣ��K=_________________��
(4)����(3)��ʵ����30minʱ��ʼ���£�36minʱ��ƽ�⣬���NO��ת���ʱ�Ϊ50%����÷�Ӧ����H_______0����������������������=�������жϵ�������___________________��
(5)����ѧ����30min��ı���ijһ��������Ӧ���е�40minʱ��ƽ��Ũ�ȷֱ�Ϊc��NO��=0.032mol/L��c��N2��=0.034mol/L��c��CO2��=0.017mol/L����ı������������______���жϵ�������____________________��