��Ŀ����
���Ṥҵβ���е��������NO��NO2���Ǵ�����Ⱦ����û������з�Ӧ�ķ���������2NO2+2NaOH====NaNO2+NaNO3+H2O
NO2+NO+2NaOH====2NaNO2+H2O
��1�����ڱ�״������NO��NO2�Ļ����ǡ����50 mL 2.0 mol��L-1��NaOH��Һ��Ӧ��ȫ��������NaNO2��NaNO3�����ʵ����ı�ֵΪ4��1�����ڻ��������NO������������Ϊ���٣�
��2����NO��NO2�Ļ��������NOx��ʾ���ü�Һ���գ����������Ƽ����������ٽᾧ���롣������ÿ�������Ƶijɱ�Ϊ0.16��Ԫ������ÿ���������Ƶijɱ�Ϊ0.27��Ԫ��Ŀǰ�г����ۼۣ�������ÿ��0.18��Ԫ����������ÿ��0.28��Ԫ����ÿ����22.4��109 L����״������NOx��x��1.5��0.1%�������������β������������y�����ۼۼ�ȥ�ɱ��ۣ���λ����Ԫ����x�Ĺ�ϵΪ______________������������̣�
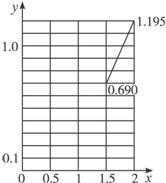
��3�����ݣ�2���ļ�����������������ϵ��������ʾ��ͼ��
��1��NO��NO2���ʵ���֮����֮��Ӧ��NaOH���ʵ�����ȣ�Ϊ0.1 mol����NO2��NO�����ʵ����ֱ�Ϊa��b��
2NO2+2NaOH====NaNO2 + NaNO3+H2O
2 2 1 1
(a-b) (a-b) 0.5(a-b) 0.5(a-b)
NO2+NO+2NaOH====2NaNO2+H2O
1 1 2 2
b b 2b 2b
![]()
a=0.07 mol b=0.03 mol
����������NO�����������0.03 mol/��0.03 mol+0.07 mol��=3/10
��2������2NOx+2NaOH====(2x-3)NaNO3+(5-2x)NaNO2+H2O
n(NOx)=106 mol����:n(NaNO2)=0.5��(5-2x)��106 mol
n(NaNO3)=0.5��(2x-3)��106 mol
y=0.02 ��Ԫ/t��0.5��(2x-3)��106 mol��85 g��mol-1��10-6 t/g+0.01 ��Ԫ/t��0.5��(5-2x)��106mol��69 g��mol-1��10-6 t/g=��1.01x-0.825����Ԫ
��3��?

��������1����NaNO3��Ϊ1������2NO2![]() NaNO2��NaNO2������3NaNO2����1.5 NO2��1.5 NO���ɵģ�NO���������
NaNO2��NaNO2������3NaNO2����1.5 NO2��1.5 NO���ɵģ�NO���������![]()
��2���ܷ�Ӧ����ʽ��?
2NOx+2NaOH====(2x-3)NaNO3+(5-2x)NaNO2+H2O
2 2x-3 5-2x
106 mol n(NaNO3) n(NaNO2)
n(NaNO2)=![]() ��106 mol
��106 mol
n(NaNO3)=![]() ��106 mol
��106 mol
y=![]() ��106 mol��69 g��moL-1��10-6 t��g-1��0.01 ��Ԫ/t+
��106 mol��69 g��moL-1��10-6 t��g-1��0.01 ��Ԫ/t+![]() ��106 mol��85 g��mol-1��10-6 t��g-1��0.02��Ԫ/t=��1.01x-0.825����Ԫ
��106 mol��85 g��mol-1��10-6 t��g-1��0.02��Ԫ/t=��1.01x-0.825����Ԫ
��3����x=1.5ʱ��y=0.690
��x=2ʱ��y=1.195