��Ŀ����
ijУ��ѧ�о���ѧϰС����������˽�������ݣ��Ҷ���(HOOC-COOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
(1)��ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ____________________________��
(2)��ʢ���Ҷ��ᱥ����Һ���Թ��е��뼸�������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ����˵���Ҷ������_____________(������ԡ�������ԭ�ԡ������ԡ�)��
������ƽ�÷�Ӧ�����ӷ���ʽ�� ____ MnO4- + ___ H2C2O4 + ____ H+ = ____ Mn2+ + ___ CO2�� + ____H2O
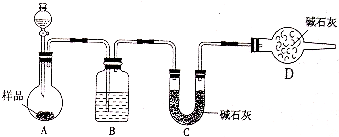
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(�г�װ��δ���)��
(1)��ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ____________________________��
(2)��ʢ���Ҷ��ᱥ����Һ���Թ��е��뼸�������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ����˵���Ҷ������_____________(������ԡ�������ԭ�ԡ������ԡ�)��
������ƽ�÷�Ӧ�����ӷ���ʽ�� ____ MnO4- + ___ H2C2O4 + ____ H+ = ____ Mn2+ + ___ CO2�� + ____H2O
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(�г�װ��δ���)��

ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ��졣�ݴ˻ش�
������װ���У�D��������__________________�����Ҷ���ֽ�Ļ�ѧ����ʽΪ_____________________________________��
(4)��С��ͬѧ��2.52 g���ᾧ��(H2C2O4��2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����___________________________________(�����ּ���)������Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ��___________________�������ӷ��ű�ʾ����
������װ���У�D��������__________________�����Ҷ���ֽ�Ļ�ѧ����ʽΪ_____________________________________��
(4)��С��ͬѧ��2.52 g���ᾧ��(H2C2O4��2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����___________________________________(�����ּ���)������Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ��___________________�������ӷ��ű�ʾ����
(1)HCO3- + H2C2O4 = HC2O4-+ CO2��+ H2O
(2) �ٻ�ԭ�� ��2��5��6��2��10��8
(3) �ٳ�ȥ��������е�CO2��H2C2O4 H2O+CO��+CO2��
H2O+CO��+CO2��
(4)��Ӧ������ҺΪNaHC2O4��Һ������HC2O4-�ĵ���̶ȱ�ˮ��̶ȴ�����Һ��c(H+) > c(OH-)��������Һ������
(5)Na+>HC2O4->H+> C2O42->OH-
(2) �ٻ�ԭ�� ��2��5��6��2��10��8
(3) �ٳ�ȥ��������е�CO2��H2C2O4
 H2O+CO��+CO2��
H2O+CO��+CO2�� (4)��Ӧ������ҺΪNaHC2O4��Һ������HC2O4-�ĵ���̶ȱ�ˮ��̶ȴ�����Һ��c(H+) > c(OH-)��������Һ������
(5)Na+>HC2O4->H+> C2O42->OH-

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д�
�����Ŀ

 ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������
ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������