��Ŀ����
��ʾ��ͼ�еļ���װ�ÿ��Խ�������ķ������ռ���
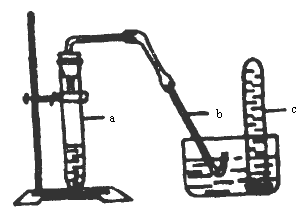
(1)ʵ��ǰӦ��μ���װ�õ������ԣ�
��________��
(2)�����Թ�a��Ƥ��������10mL 6mol��L��1��ϡ�����1g��ͭƬ�����������е��ܵ���Ƥ�������Թܿڣ���Ӧ��ʼʱ�ٶȻ��������ӿ죮��д�����Թ�a�������������з�Ӧ�Ļ�ѧ����ʽ��
��________��
(3)�ӷ�Ӧ��ʼ����Ӧ������Ԥ�����Թ�a�пɹ۲쵽��Щ������������һд����
��________��
(4)�ӷ�Ӧ��ʼʱ���ɹ۲쵽����b�е�ˮ�����ص���b����������һ���߶ȣ��˺��ֻ��䣬Ȼ�������ݴӹܿ�ð������˵����Ӧ��ʼʱ��������ˮ����������ԭ��
��________��
(5)�Թ�c�ռ����������Ĵָ��ס�ܿڣ��Ƴ�ˮ�ۣ����ܿ����ϣ��ɿ�ĴָƬ�̺��ٴζ�ס�ܿڣ����Թ����ٵ�����ˮ���У��ɿ�Ĵָ����ʱ�ɹ۲쵽ʲô����
��________��
������
|
����(1)�ѵ��ܵ��¶˽���ˮ�У����ֽ��������Թ�a�����ܿڻ�������ð�����ɿ��ֺ�ˮ�ֻ����������b�У� ����(2)��3Cu��8HNO3 ������2NO��O2��2NO2 ����(3)CuƬ���ܽ⣬����ð������Һ�������������Թ��ϲ��ռ��Ϊ����ɫ���ֱ�Ϊ��ɫ�� ����(4)���ڷ�Ӧ��ʼʱ������NO���Թ�a�ϲ������е�O2��������NO2��NO2��ˮ��Ӧ��ʹװ������ѹ��ʱ��С�����Ե����е�ˮ�����������һ���߶ȣ� ����(5)��ˮ�����Թܣ�������һ���߶ȣ� �������Թ����������ɫ�ɺ���ɫ��Ϊ��ɫ�� |

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�


 ��1��ʵ��ǰӦ��μ���װ�õ������ԣ�
��1��ʵ��ǰӦ��μ���װ�õ������ԣ�  ��1���ο��Թ�a����Ƥ��������10mL6mol/Lϡ�����1g��ͭƬ�����������е��ܵ���Ƥ�������Թܿڡ���Ӧ��ʼʱ�ٶȻ����������ӿ졣��д�����Թ�a�������������з�Ӧ�Ļ�ѧ����ʽ��
��1���ο��Թ�a����Ƥ��������10mL6mol/Lϡ�����1g��ͭƬ�����������е��ܵ���Ƥ�������Թܿڡ���Ӧ��ʼʱ�ٶȻ����������ӿ졣��д�����Թ�a�������������з�Ӧ�Ļ�ѧ����ʽ��