��Ŀ����
��������Ȼ�ѧ����ʽ����ѧ����ʽ���缫��Ӧʽ������ʽ�ȣ�����д��
��1����֪��2Cu(s)��1/2O2(g)=Cu2O(s)����H = -169kJ��mol-1��
C(s)��1/2O2(g)=CO(g)����H = -110.5kJ��mol-1��
Cu(s)��1/2O2(g)=CuO(s)����H = -157kJ��mol-1
��̿���ڸ��������»�ԭCuO����Cu2O���Ȼ�ѧ����ʽ�ǣ�
��2����һ�������£���������������������·�Ӧ��2SO2(g)+O2(g) 2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
��3���Լ��顢����Ϊ��Ӧ�KOH��Һ���������Һ����ȼ�ϵ�أ�����ӦʽΪ�� ��
��4����ˮAlCl3ƿ�Ǵ��а������䷴Ӧ�Ļ�ѧ����ʽΪ ��
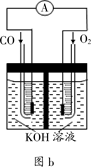
��5����þ���������Ρ�ȼ�ϵ�أ���װ��ʾ��ͼ��ͼ���õ�ط�Ӧ���ܷ�Ӧ����ʽΪ_____________________��
��6����ҵ�ϵ�ⱥ��ʳ��ˮ�����ӷ���ʽΪ________________��
��12�֣���1��2CuO(s)��C(s)= Cu2O(s)��CO(g)����H ="+34.5" kJ��mol-1 ��2�֣�
��2��K= c2(SO3)/ c2(SO2) c (O2) ��2�֣�
��3��CH4+10OH����8e��=CO32��+7H2O�� ��2�֣�
��4��AlCl3��3H2O Al(OH)3��3HCl ��2�֣�
Al(OH)3��3HCl ��2�֣�
��5��Mg+ClO-+ H2O= Cl-+Mg(OH)2 ��2�֣�
��6��2Cl����2H2O 2OH����Cl2����H2�� ��2�֣�
2OH����Cl2����H2�� ��2�֣�
���������������1����֪����2Cu��s��+1/2O2��g��=Cu2O��s������H=-169kJ��mol-1��
��C��s��+1/2O2��g��=CO��g������H=-110.5kJ��mol-1��
��Cu��s��+1/2O2��g��=CuO��s������H=-157kJ��mol-1
�ɸ�˹���ɿ�֪����-�ۡ�2+�ڵ�2CuO��s��+C��s��=Cu2O��s��+CO��g������H=-169kJ��mol-1-��-157kJ��mol-1����2=-110.5kJ��mol-1="+34.5" kJ��mol-1��
�ʴ�Ϊ��2CuO��s��+C��s��=Cu2O��s��+CO��g������H="+34.5" kJ��mol-1��
��2�����淴Ӧ2SO2��g��+O2��g�� 2SO3��g���Ļ�ѧƽ�ⳣ��
2SO3��g���Ļ�ѧƽ�ⳣ��
�ʴ�Ϊ��
��3��ԭ��ظ�������������Ӧ�������ڸ����ŵ磬������������Ӧ�����������������ŵ��������������ӣ������缫��ӦʽΪ2O2+4H2O+8e��=8OH�����ܵĵ�ط�ӦʽΪCH4+2O2+2OH��=CO32��+3H2O������ܷ�Ӧʽ��ȥ�����缫��Ӧʽ�ɵø����缫��ӦʽΪCH4+10OH��-8e��=CO32��+7H2O��
�ʴ�Ϊ��CH4+10OH��-8e��=CO32��+7H2O��
��4��AlCl3ˮ��AlCl3+3H2O Al��OH��3+3HCl����HCl���Ȼ���������е�ˮ�����ʰ�����
Al��OH��3+3HCl����HCl���Ȼ���������е�ˮ�����ʰ�����
�ʴ�Ϊ��AlCl3+3H2O Al��OH��3+3HCl��
Al��OH��3+3HCl��
��5����ͼ��֪þ-�������Ρ�ȼ�ϵ����Mg��ClO����H2O��Ӧ����Cl����Mg��OH��2���õ�ط�Ӧ���ܷ�Ӧ����ʽΪMg+ClO��+H2O=Cl��+Mg��OH��2��
�ʴ�Ϊ��Mg+ClO��+H2O=Cl��+Mg��OH��2��
��6����ⱥ��ʳ��ˮ�����������������������ƣ���ⱥ��ʳ��ˮ�����ӷ���ʽΪ2Cl��+2H2O 2OH��+Cl2��+H2����
2OH��+Cl2��+H2����
�ʴ�Ϊ��2Cl��+2H2O 2OH��+Cl2��+H2��
2OH��+Cl2��+H2��
���㣺�ø�˹���ɽ����йط�Ӧ�ȵļ��㣻ԭ��غ͵��صĹ���ԭ������ѧƽ�ⳣ���ĺ���

�������������ʡ�����ȵ���Ҫԭ�ϣ�Χ�ƺϳɰ����ǽ�����һϵ�е��о�
��1�����������뵪������������������Ӧ�����Ƿ�Ӧ������ȴ����ͬ��
��֪��2H2 (g) + O2 (g) = 2H2O (g) ��H =" -483.6" kJ/mol
3H2 (g) + N2 (g)  2NH3 (g) ��H =" -92.4" kJ/mol
2NH3 (g) ��H =" -92.4" kJ/mol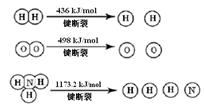
�������1 mol N��N����Ҫ���� kJ �� ���������л�ѧ�������������еĻ�ѧ���� ���ǿ�����������������������߷�Ӧ��������ͬ��
��2���̵��ǿ�ѧ�������о�����Ҫ���⡣��Ȼ���д�����Ȼ�Ĵ����̵����̣�N2 (g) + O2 (g) =" 2NO" (g) ��H =" +180.8" kJ/mol ����ҵ�ϳɰ������˹��̵���
�������̵ֹ���Ӧ��ƽ�ⳣ�������н�����ȷ���� ��
| ��Ӧ | �����̵� | ��ҵ�̵� | ||||
| �¶�/�� | 27 | 2000 | 25 | 350 | 400 | 450 |
| K | 3.84��10-31 | 0.1 | 5��108 | 1.847 | 0.507 | 0.152 |
A�������£������̵����������ܽ��У�����ҵ�̵��dz�������
B��������ģģ������̵����������
C����ҵ�̵��¶�Խ�ͣ�������������ӦԽ��ȫ
D��KԽ��˵���ϳɰ���Ӧ������Խ��
��3���ں��º����ܱ������а��ռס��ҡ������ַ�ʽ�ֱ�Ͷ��, ������Ӧ��3H2 (g) + N2 (g)
 2NH3 (g)��ü�������H2��ת����Ϊ40%��
2NH3 (g)��ü�������H2��ת����Ϊ40%��| | N2 | H2 | NH3 |
| �� | 1 | 3 | 0 |
| �� | 0.5 | 1.5 | 1 |
| �� | 0 | 0 | 4 |
��ƽ��ʱ���ס��ҡ�����������NH3�����������С˳��Ϊ ��
��4�������Ǻϳ������ԭ�ϣ�д��������������Ӧ����һ����������̬ˮ���Ȼ�ѧ����ʽ ��
�о����仯�������������Ҫ���塣
��1��Cu2S�ڸ��������·������·�Ӧ��
2Cu2S(s)+3O2(g)=2Cu2O(s)+2SO2(g) �SH=��773kJ/mol
���÷�Ӧ��1.2mol����ת��ʱ,��Ӧ�ͷų�������Ϊ kJ��
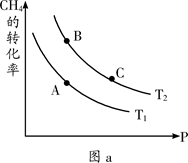
��2�����Ṥҵ�������漰��Ӧ��2SO2(g)+O2(g) 2SO3(g)��SO2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
2SO3(g)��SO2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��ѹǿ��P1 P2�����������=����<������
��ƽ�ⳣ����A�� B�㣨���������=����<������
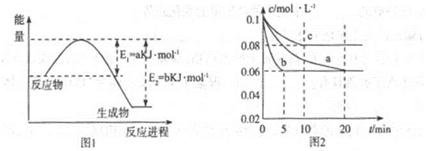
��200���£���һ������SO2��O2�������������ܱ������У���10min���������и����ʵ����ʵ���Ũ�����±���ʾ:
| ���� | SO2 | O2 | SO3 |
| Ũ�ȣ�mol/L�� | 0.4 | 1.2 | 1.6 |
��˵���÷�Ӧ�ﵽ��ѧƽ��״̬���� ��
a��SO2��O2������ȱ��ֲ���
b����ϵ��ѹǿ���ֲ���
c�����������ܶȱ��ֲ���
d��SO2��SO3���ʵ���֮�ͱ��ֲ���
����������Ӧ��0~10min�ڣ���(O2)= ��
��3��һ���¶��£���ˮ����SO2����ʱ����Һ��ˮ�ĵ���ƽ�� �ƶ�����������ҡ������������õ�pH=3��H2SO3��Һ���Լ�����Һ��
 ������֪���¶���H2SO3�ĵ��볣����Ka1=1.0��10-2 mol/L��Ka2=6.0��10-3 mol/L��
������֪���¶���H2SO3�ĵ��볣����Ka1=1.0��10-2 mol/L��Ka2=6.0��10-3 mol/L�� ������������Ȼ����Ϊԭ�Ϻϳɼ״������ⱻһһ���ˣ�����شٽ��˼״���ѧ�ķ�չ��
��1����̿��ˮ�����ķ�Ӧ���ƣ�����Ȼ��Ϊԭ��Ҳ�����Ƶ�CO��H2���÷�Ӧ�Ļ�ѧ����ʽΪ_________��
��2���ϳɼ״���һ�ַ�������CO��H2Ϊԭ�ϣ��������仯��ͼ��ʾ��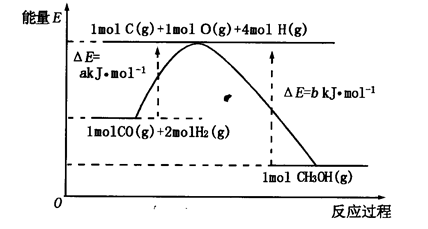
��ͼ��֪���ϳɼ״����Ȼ�ѧ����ʽΪ________________________________________��
��3����CO2Ϊԭ��Ҳ���Ժϳɼ״����䷴Ӧԭ��Ϊ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
����lL���ܱ������У�����1molCO2��3molH2����500���·�����Ӧ�����CO2(g)��CH3OH(g)��Ũ����ʱ�ʱ仯��ͼ��ʾ��
������˵����ȷ����_________________(����ĸ)��
| A��3minʱ��Ӧ�ﵽƽ�� |
| B��0��10minʱ��H2��ʾ�ķ�Ӧ����Ϊ0��225mol��-1��min-1 |
| C��CO2��ƽ��ת����Ϊ25�� |
D�����¶�ʱ��ѧƽ�ⳣ��Ϊ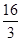 ��mol/L����2 ��mol/L����2 |
| ���� | ����1 | ����2 | ����3 |
| ��Ӧ��Ͷ������ʼ̬�� | 1molCO2��3molH2 | 0.5molCO2��1.5molH2 | 1molCH3OH��1molH2O |
| CH3OH��ƽ��Ũ��/mol?L-1 | c1 | c2 | c3 |
| ƽ��ʱ��ϵѹǿ/Pa | p1 | p2 | p3 |
�����и����Ĵ�С��ϵΪc1___________c3��p2_________p3(����ڡ��������ڡ���С�ڡ�)��
��4�����������״�ȼ�ϵ�ؼ���������µ�ͻ�ƣ���ͼ��ʾΪ�״�ȼ�ϵ�ص�װ��ʾ��ͼ����ع���ʱ���ֱ��b��c����CH3OH��O2���ش��������⣺

�ٴ�d���ų���������___________����Һ�е���������缫__________(�M����N��)��
�ڵ缫M�Ϸ����ĵ缫��ӦʽΪ__________________________��
 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 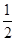 O2(g)�TCO2(g) ��H����283.0kJ/mol
O2(g)�TCO2(g) ��H����283.0kJ/mol CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��
CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)�� 2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
 2NH3(g)��H<0
2NH3(g)��H<0
 CO(g) + 3H2(g) ��H>0
CO(g) + 3H2(g) ��H>0