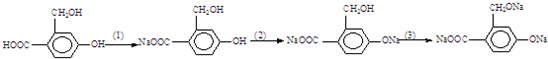��Ŀ����
��1���밴Ҫ����д��ѧ����ʽ��������ͭ���뷽��ʽ��_______________��
������ͭˮ������ӷ���ʽ��_______________��
������ͭ��Һ������������Ӧ�����ӷ���ʽ��_______________��
���ò��缫�������ͭ��Һ�ĵ缫��Ӧʽ��������____________��������_______________�����Ļ�ѧ����ʽ______________________________��
��2������NaHSO4��Һ�У���μ���Ba(OH)2��Һ�����ԣ�д��������Ӧ�����ӷ���ʽ______________________________����������������Һ�У������μ�Ba(OH)2��Һ��д���˲���Ӧ�����ӷ���ʽ��______________________________��
����Ba(OH)2��Һ�У���μ���NaHSO4��Һ�����ԣ�д��������Ӧ�����ӷ���ʽ��______________________________________����������Һ�У������μ�NaHSO4��Һ��д���˲���Ӧ�����ӷ���ʽ��______________________________��
����Ba(OH)2��Һ�У���μ���������Һ��Ba2+ǡ����ȫ�������䷴Ӧ�����ӷ���ʽ��______________________________����������Һ�У�������������Һ����д���˲���Ӧ�����ӷ���ʽ��______________________________��
��������1��Ӧ��ע���������෴Ӧ����ʽ��д���ϵIJ�ͬ�����в��缫Ϊ���Ե缫������ܷ�Ӧ�ɽ�������Ӧ��������Ӧ�ϲ�������
��2��Ba(OH)2��NaHSO4�ķ�Ӧ�����⣬Ӧ���ע���ж��ڷ�Ӧ���������ʹ�����Ba(OH)2�������ķ�Ӧ����Ba2+��![]() ��OH-��Al3+��������Ӧ�����жϷ�Ӧ��������ʱӦͬʱ���ǡ�
��OH-��Al3+��������Ӧ�����жϷ�Ӧ��������ʱӦͬʱ���ǡ�
�𰸣���1����CuSO4====Cu2++![]()
��Cu2++2H2O![]() Cu(OH)2+2H+
Cu(OH)2+2H+
��Cu2++![]() +Ba2++2OH-====BaSO4��+Cu(OH)2��
+Ba2++2OH-====BaSO4��+Cu(OH)2��
��Cu2++2e-====Cu
4OH--4e-====2H2O+O2��
2CuSO4+2H2O![]() 2Cu+O2��+2H2SO4
2Cu+O2��+2H2SO4
��2����2H++![]() +Ba2++2OH-====BaSO4��+2H2O
+Ba2++2OH-====BaSO4��+2H2O
Ba2++![]() ==== BaSO4��
==== BaSO4��
��Ba2++OH-+![]() ==== BaSO4��+H2O OH-+H+====H2O
==== BaSO4��+H2O OH-+H+====H2O
��2Ba2++4OH-+2![]() +Al3+====2BaSO4��+
+Al3+====2BaSO4��+![]() +2H2O
+2H2O
Al3++3![]() +6H2O====4Al(OH)3��
+6H2O====4Al(OH)3��

 ��2010?�Ĵ����ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺
��2010?�Ĵ����ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺
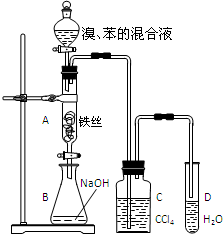 ij��ѧ����С������ͼװ����ȡ�屽��̽���÷�Ӧ�����ͣ������Һ©���м��뱽��Һ�壬�ٽ����Һ���뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С������ͼװ����ȡ�屽��̽���÷�Ӧ�����ͣ������Һ©���м��뱽��Һ�壬�ٽ����Һ���뷴Ӧ��A��A�¶˻����رգ��У�

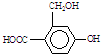 Ϊʵ���������ʵ�ת��
Ϊʵ���������ʵ�ת��