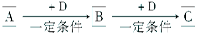��Ŀ����
(12��)��ѧ��ѧ�г����ļ������ʴ������¹�ϵ�����м��Ǻ�ɫ�ǽ������ʣ����������г����Ľ������ʣ�C�ڳ�����Ϊ��ɫ��Һ�壬D�Ǻ���ɫ���塣��ͼ�в��ֲ���ͷ�Ӧ��������ȥ��

�ش��������⣺
(1)д������A��Ũ��Һ��Ӧ�Ļ�ѧ����ʽ_________________________________��
(2)C�ĵ���ʽ��___________________________��
(3)��ȥG�����к���H���ʲ��õķ�����_____________________��
(4)A��Һ��һ����ʹʪ��ĺ�ɫʯ����ֽ���������巴Ӧ������һ���Σ����ε���Һ�����ԣ���ԭ���ǣ������ӷ���ʽ��ʾ��_____________________________��
(5)д����Fת��ΪE�����ӷ���ʽ____________________________________��
(6)д����Gת��ΪH�����ӷ���ʽ____________________________________��
��12�֣���1��C+4HNO3��Ũ��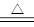 CO2��+4NO2��+2H2O ��2�֣�
CO2��+4NO2��+2H2O ��2�֣�
��2�� ��2�֣�
��3�����ȣ�2�֣�
��2�֣�
��3�����ȣ�2�֣�
��4��NH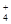 + H2O
+ H2O NH3��H2O + H+ ��2�֣�
NH3��H2O + H+ ��2�֣�
��5��2Fe3+ ��Fe 3Fe2+��2�֣�
3Fe2+��2�֣�
��6��CO32- +H2O +CO2=2HCO3-��2�֣�
���������ۺϸ����ʵ���ɫ״̬�������ʵ�ת����ϵ�����ж���Ϊ̼��AΪ���
C+4HNO3��Ũ��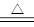 CO2��+4NO2��+2H2O ��2�֣����е�DΪNO2��CΪ��2�ϡ�BΪCO2��
CO2��+4NO2��+2H2O ��2�֣����е�DΪNO2��CΪ��2�ϡ�BΪCO2��
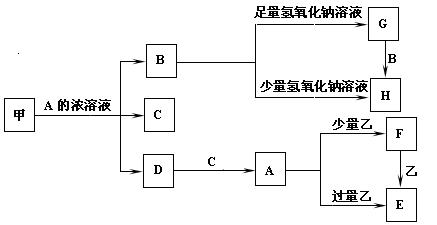
����ͽ����ҵIJ������ҵ����Ƕ����йأ���֪��Ϊ��۽������ɲ¶�Ϊ����

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�