��Ŀ����
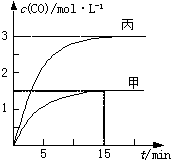 һ�������´��ڷ�Ӧ��C��s��+H2O��g���TCO��g��+H2��g����H��0����ס��ҡ����������������м���һ����C��H2O�����������¶ȡ���Ӧ�����ʼ�����±�����Ӧ������CO�����ʵ���Ũ����ʱ��仯��ͼ��
һ�������´��ڷ�Ӧ��C��s��+H2O��g���TCO��g��+H2��g����H��0����ס��ҡ����������������м���һ����C��H2O�����������¶ȡ���Ӧ�����ʼ�����±�����Ӧ������CO�����ʵ���Ũ����ʱ��仯��ͼ��| ���� | �� | �� | �� |
| �ݻ� | 0.5L | 0.5L | V |
| �¶� | T1�� | T2�� | T1�� |
| ��ʼ�� | 2molC 1molH2O |
1molCO 1molH2 |
4molC 2molH2O |
| A���������У���Ӧ��ǰ15 min��ƽ������v��H2��=0.1 mol?L-1?min-1 |
| B�������������V��0.5 L |
| C�����¶�ΪT1��ʱ����Ӧ��ƽ�ⳣ��K=2.25 |
| D���������У���ƽ��ʱn��H2O��=0.4 mol����T1��T2 |
������A����ͼ��֪��15min�ڼ�������CO��Ũ�ȱ仯��Ϊ1.5mol/L������v=
����v��CO��������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��H2����
B������������ʼ��Ϊ�Ķ��������ݻ�=0.5 L����������ӦΪ�����������ķ�Ӧ����ѹƽ�����ƣ�c��CO����3mol/L��
C�����ݼ������з�Ӧ���ݼ��� T1��ʱ����Ӧ��ƽ�ⳣ��K=
=
=4.5��
D���Ƚϼ����ҿ�֪�����ߴ��Чƽ�⣬���ݼס���������ƽ��ʱn��H2O��������֪��������ڼ�ƽ�����淴Ӧ�ƶ�����Ϊ����Ӧ���ȣ������¶ȵͣ����¶�T1��T2��
| ��c |
| ��t |
B������������ʼ��Ϊ�Ķ��������ݻ�=0.5 L����������ӦΪ�����������ķ�Ӧ����ѹƽ�����ƣ�c��CO����3mol/L��
C�����ݼ������з�Ӧ���ݼ��� T1��ʱ����Ӧ��ƽ�ⳣ��K=
| c(CO)c(H2) |
| c(H2O) |
| 1.5��1.5 |
| 0.5 |
D���Ƚϼ����ҿ�֪�����ߴ��Чƽ�⣬���ݼס���������ƽ��ʱn��H2O��������֪��������ڼ�ƽ�����淴Ӧ�ƶ�����Ϊ����Ӧ���ȣ������¶ȵͣ����¶�T1��T2��
����⣺A����ͼ��֪��15min�ڼ�������CO��Ũ�ȱ仯��Ϊ1.5mol/L��v��CO��=
=0.1mol?L-1?min-1������֮�ȵ��ڻ�ѧ������֮�ȣ�����v��H2��=0.1mol?L-1?min-1����A��ȷ��
B������������ʼ��Ϊ�Ķ��������ݻ�=0.5 L����������ӦΪ�����������ķ�Ӧ����ѹƽ�����ƣ�c��CO����3mol/L����C���������V��0.5 L����B��ȷ��
C�����ݼ������з�Ӧ���ݼ��㣺
C��s��+H2O��g���TCO��g��+H2��g��
��ʼŨ�� 2 0 0
ת��Ũ�� 1.5 1.5 1.5
ƽ��Ũ�� 0.5 1.5 1.5
T1��ʱ����Ӧ��ƽ�ⳣ��K=
=
=4.5����C����
D���Ƚϼ����ҿ�֪�����ߴ�ƽ���ǵ�Ч�ģ�������֪��������ƽ��ʱn��H2O��=0.25 mol���������У���ƽ��ʱn��H2O��=0.4 mol��������ڼ�ƽ�����淴Ӧ�ƶ�����Ϊ����Ӧ���ȣ������¶ȵͣ����¶�T1��T2����D����
��ѡAB��
| 1.5mol/L |
| 15min |
B������������ʼ��Ϊ�Ķ��������ݻ�=0.5 L����������ӦΪ�����������ķ�Ӧ����ѹƽ�����ƣ�c��CO����3mol/L����C���������V��0.5 L����B��ȷ��
C�����ݼ������з�Ӧ���ݼ��㣺
C��s��+H2O��g���TCO��g��+H2��g��
��ʼŨ�� 2 0 0
ת��Ũ�� 1.5 1.5 1.5
ƽ��Ũ�� 0.5 1.5 1.5
T1��ʱ����Ӧ��ƽ�ⳣ��K=
| c(CO)c(H2) |
| c(H2O) |
| 1.5��1.5 |
| 0.5 |
D���Ƚϼ����ҿ�֪�����ߴ�ƽ���ǵ�Ч�ģ�������֪��������ƽ��ʱn��H2O��=0.25 mol���������У���ƽ��ʱn��H2O��=0.4 mol��������ڼ�ƽ�����淴Ӧ�ƶ�����Ϊ����Ӧ���ȣ������¶ȵͣ����¶�T1��T2����D����
��ѡAB��
���������⿼�黯ѧƽ���ƶ������㼰��ѧƽ��ͼ���Ѷ��еȣ�ע������ͼ���������ͺ���������壮

��ϰ��ϵ�д�
 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
�����Ŀ
��һ�������´��ڷ�Ӧ��I2��g��+H2��g��?2HI��g����������Ӧ���ȣ�����3����ͬ��2L���ݾ��ȣ������û�������������ܱ������ס��ҡ������ڼ��г���1mol I2��g����1mol H2��g���������г���2mol HI��g�����ڱ��г���2mol I2��g����2mol H2��g������һ���¶����¿�ʼ��Ӧ���ﵽƽ��ʱ������˵������ȷ���ǣ�������
| A�������ס����У��淴Ӧ�����ʣ�v���ף�=v������ | B�������ס����У�HI �����ʵ�����n���ף���n���ң� | C�������ס������йط�Ӧ���ת���ʣ�����H2��+����HI����100% | D�������ס����У���ѧƽ�ⳣ����K���ף�=K���ң� |
һ�������´��ڷ�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����������Ӧ���ȣ�����������ͬ��2L���ݾ��ȣ������û�������������ܱ��������ڢ��г���1mol CO��1mol H2O���ڢ��г���1mol CO2��1mol H2���ڢ��г���2mol CO ��2mol H2O��700�������¿�ʼ��Ӧ���ﵽƽ��ʱ������˵����ȷ���ǣ�������
| A��������������Ӧ������ͬ | B���������з�Ӧ��ƽ�ⳣ����ͬ | C����������CO�����ʵ������������еĶ� | D����������CO��ת��������������CO2��ת����֮�͵���1 |