��Ŀ����
Ϊ�ⶨ̼��ƴ��ȣ��躬����SiO2����ѧ����������¼���ʵ�鷽������ش�ÿ�������е����⡣
������
�ٳ�ȡ̼�����Ʒmg��
�ڼ���������
���ռ����ⶨ���ɵ��������VmL��
���⣺���������Ʒʱ������ײ���һ��δ���˷��ֵIJ�ȱ����ô��õ�̼��ƴ��Ȼ�________��ƫ�ߡ�ƫ�͡���Ӱ�죩��
������
�ٳ�ȡ̼�����Ʒmg��
����c mol•L��1����VmL���������ܽ���Ʒ��
��ȡ�ܽ�����Һ![]() mL����c��mol•L��1 NaOH��Һ�ζ�����ȥV��mL��
mL����c��mol•L��1 NaOH��Һ�ζ�����ȥV��mL��
���⣺��1���г���ʵ�������õ����������ƣ�������̨���ջ�������̨�����⣩________��
��2����������Ƿ���Ҫ�˳�SiO2������NaOH�ζ�________����ѡ���ţ���
A����Ҫ�������������������������������� B������Ҫ������������������������ C������
��3��̼��ƴ��ȼ��㹫ʽ________��
������
�ٳ�ȡ̼�����Ʒmg��
�۸�������1000��ֱ���������ٸı䣬��ȴ�����������Ϊm��g��
���⣺��1��ΪʲôҪ������1000���ҡ�ֱ���������ٸı䡱��
��2���������еġ���ȴ����β�����Ϊʲô��
��������
�ٳ���̼�����Ʒmg��
�ڼ�������c mol•L��1����VmLʹ֮��ȫ�ܽ⣻
�۹��˲�ȡ��Һ��
������Һ�м������c��mol•L��1Na2CO3��ҺV��mL��
�ݽ�������еij����˳���ϴ�ӡ��������Ϊm��g��
���⣺��1���˷����в���Ҫ��������________����ѡ���ţ���
A��c�䡢V�������� ���� B��c�䡢V������ ���� C��m�� ���������������� D��m
��2��Ϊ����ʵ������Ҫ�IJ�����________����ѡ���ţ���
A����ȷ�ⶨNa2CO3��Һ���V��mL
B����ȷ����Ũ��c��mol•L��1Na2CO3��Һ
C������������ó���ϴ�ӡ�ϴҺҲӦ�������
D������������ó���ϴ�ӡ��������������m��g����
��3���������Ҫ����������ϴ�ӣ����δ��ϴ�ӣ���ⶨ�����̼��ƴ��Ƚ�________��ƫ�ߡ�ƫ�͡���Ӱ�죩��
��������������Ϊ�ĸ������У���õķ�����________
����������ȱ��ֱ��ǣ�
��������������ϴ�ӡ���������������̸��ӣ�������ɽϴ���
������________��
������________��
������
������ƫ�� ������1��������ƽ��ҩ�ס��ᡢ��ʽ�ζ��ܡ���ƿ����2��B����3�� ������1����֤CaCO3��ȫ�ֽ⡣ ��2��Ӧ�ڸ���������ȴ����ֹ���ɵ�CaO�������CO2��H2O��Ӧ����������ı�������� ����������1��A��B ��2��C ��3��ƫ�� ��õķ����Ƿ����� ����������������Բ���ȷ����Ϊ���������������ص�Ӱ��̫�� ������ʵ�������ṩ1000��ĸ���
|
��ʾ��
�������У�����ײ��в�ȱ����ʹ������̼�����Ʒ��������ʵ�ʵ�����С��������������CO2��ʵ��С���Ӷ�������Ĵ�̼��Ƶ�����ƫС���ȵļ����з���ƫС����ĸ���䣬��Ϊmg��ʹ����ƫ�͡� �����ٸ���ʵ�鲽����ȷ��ʵ����������һ��������������������ƽ��ҩ�ס��ڶ�����������̼��Ʒ�Ӧ���������ձ������������к͵ζ���NaOH��Һ�������������ᣬ�����Ǽ�ʽ�ζ��ܣ���ʽ�ζ��ܣ�������ȡV/10mL�ķ�ӦҺ������ƿ������ΪNaOH�ڵζ�ʱ�������Ҳ��������SiO2��Ӧ����������ˡ�������3���� n(HCl)��2n(CaCO3)��10n(NaOH) n( m(CaCO3)��M(CaCO3)��n(CaCO3)=100g•mol��1��
����= ������1����֤CaCO3��ַֽ⡣��2��Ӧ�ڸ���������ȴ����ֹ���ɵ�CaO�������CO2��H2O��Ӧ����������ı�������� ����������1������=
|

 Ϊ�ⶨ̼��ƴ��ȣ�����������ΪSiO2����ͬѧ���������������ʵ�鷽����
Ϊ�ⶨ̼��ƴ��ȣ�����������ΪSiO2����ͬѧ���������������ʵ�鷽����
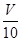 mL���Է�̪��ָʾ������c�� mol/L NaOH��Һ�ζ���ǡ����ȥV��mL��
mL���Է�̪��ָʾ������c�� mol/L NaOH��Һ�ζ���ǡ����ȥV��mL��