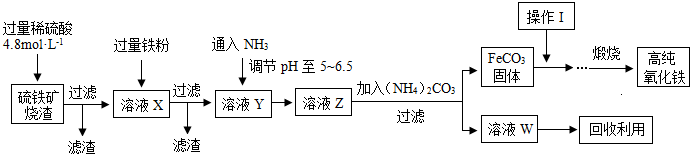
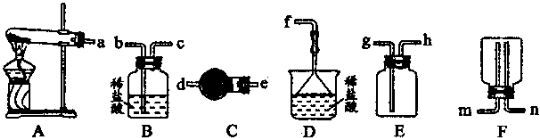
��Ŀ����
��18.4mol/L��Ũ��������100mLŨ��Ϊ1mol/L��ϡ���ᣬ���������ɷֽ�Ϊ���¼�����
A������Ͳ��ȡ5.4mL��Ũ���ᣬ����ע��װ��Լ50mL����ˮ���ձ�����ò��������裮
B����Լ30mL����ˮ���ֳ�����ϴ���ձ��Ͳ���������ÿ��ϴ��Һ����������ƿ�
C����ϡ�ͺ������С�ĵ��ò���������������ƿ��
D�����100mL����ƿ���Ƿ�����©��
E��������ˮֱ�Ӽ�������ƿ����Һ��ӽ����ο̶���2cm��3cm��
F���ǽ�ƿ���������㵹��ҡ����Һ��
G���ý�ͷ�ι�������ƿ����ε�������ˮ����Һ����͵�ǡ���뻷�ο̶������У�
�ݴ���д��
��1����ȷ�IJ���˳����______��������ţ�
��2�����в���ʱ��ѡ������ƿ�Ĺ���ǣ�����ţ�______��
A��10mL���������� B��50mL�������� C��100mL����������D��1000mL
��3������A�������ʱ�����뾭��______���ܽ��к���IJ�����
��4�������װ��Ũ�������Ͳ���Ӷ���Ϊ5.4mL�����Ƶ�ϡ�����Ũ�Ƚ�______�����ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�⣺��1�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ���ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ��ʴ�Ϊ��DACBEGF��
��2����������Һ�����Ϊ100mL����������ƿ�Ĺ��Ϊ100mL����ѡ��C��
��3����ת����ҺʱӦ����Һ���¶Ȼָ������£��ʴ�Ϊ����ȴ��
��4������Ͳ���Ӷ�������ȡ��Һ�����ƫ�����ʵ�����ƫ�࣬Ũ��ƫ�ʴ�Ϊ��ƫ�ߣ�
��������1������ʵ������IJ��������
��2������������Һ�������ѡ��������
��3��ת����ҺʱӦ����Һ���¶Ȼָ������£�
��4����Ͳ���Ӷ�������ȡ��Һ�����ƫ�����ʵ�����ƫ�࣮
���������⿼����һ�����ʵ���Ũ����Һ�����Ƶ�ʵ��������ѶȲ����ݿα�֪ʶ������ɣ�
��2����������Һ�����Ϊ100mL����������ƿ�Ĺ��Ϊ100mL����ѡ��C��
��3����ת����ҺʱӦ����Һ���¶Ȼָ������£��ʴ�Ϊ����ȴ��
��4������Ͳ���Ӷ�������ȡ��Һ�����ƫ�����ʵ�����ƫ�࣬Ũ��ƫ�ʴ�Ϊ��ƫ�ߣ�
��������1������ʵ������IJ��������
��2������������Һ�������ѡ��������
��3��ת����ҺʱӦ����Һ���¶Ȼָ������£�
��4����Ͳ���Ӷ�������ȡ��Һ�����ƫ�����ʵ�����ƫ�࣮
���������⿼����һ�����ʵ���Ũ����Һ�����Ƶ�ʵ��������ѶȲ����ݿα�֪ʶ������ɣ�

��ϰ��ϵ�д�
�����Ŀ
��18.4mol?L-1��Ũ��������4mol?L-1��������Һʱ������������������ҺŨ��ƫ�ߵ��ǣ�������
| A����ϡ�͵�������Һת��������ƿ��δϴ���ձ��Ͳ����� | B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�� | C���ý�ͷ�ιܼ�ˮʱ�������ӹ۲���Һ��Һ��������ƿ�̶����� | D���ý�ͷ�ι�������ƿ�м�ˮʱ��Һ�����������ƿ�̶ȣ���ʱ�����õιܽ�ƿ��Һ��������ʹ��Һ��Һ����̶����� |