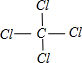��Ŀ����
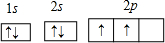
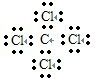
Ԫ��A��B��C���Ƕ�����Ԫ�أ����ǵ�ԭ��������Դ�СΪA��B��C��AԪ��ԭ�ӵ�����������Ϊ�����������Ķ�����BԪ�ص�ԭ�Ӵ����������������������Ķ�����B��C���γɹ��ۻ�����BC4���������(1)������Ԫ������Ӧ����̬�⻯������ȶ��Ļ�����ĵ���ʽ�ǣ�________________________________________________________________��
(2)���ǵ�����������Ӧ��ˮ������������ǿ���ǣ�________________��
(3)BC4���ȶ�����ˮ���γ�B�ĺ����ἰC���⻯�������ս��Ϊ���ڱξ���Ŀ�꣬�еIJ���BC4��Ũ��ˮ�ķ����γ���Ļ����д���йط�Ӧ�Ļ�ѧ����ʽ��_____________________��
(1) (2)HClO4
(2)HClO4
(3)SiCl4+3H2O====H2SiO3+4HCl��SiCl4+4H2O====H4SiO4+4HCl
HCl+NH3====NH4Cl
�������� ������Ԫ���ƶϿ���ԭ�ӽṹ(ԭ�Ӻ�������Ų�����)������ʽ��֪ʶ�����е�ƫ���⡣

��ϰ��ϵ�д�
 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ