��Ŀ����
��1����֪ij������������ٷ����Ϊ80.0%CH4��15.0%C2H4��5.00%C2H6�������0.500Ħ�û������������ͱ�״���µ��ܶȣ���/������
��2��CH4��һ�������´�������������C2H4��C2H6��ˮ��������Ӧ������Բ��ƣ���ȡһ����CH4����������õ�һ�ֻ�����壬���ڱ�״���µ��ܶ�Ϊ0.780��/������֪��Ӧ��CH4������20.0%��������������C2H4������ٷֺ��������������������뱣��3λ��Ч���֣�
�⣺��1��0.500Ħ�û�����������=0.500mol����16g/mol��0.800+28g/mol��0.150+30g/mol��0.0500��=9.25g��
���������ܶ�=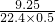 =0.826��g/l����
=0.826��g/l����
��0.500Ħ�û�����������Ϊ9.25g�ͱ�״���µ��ܶ�Ϊ0.826g/l��
��2���跴Ӧ֮ǰ��������ʵ���Ϊ1mol��������xmolת��ΪC2H4��������0.5xmolC2H4����0.5��0.200-x��mol���飬
��Ӧ��������������ʵ���=0.800mol+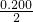 mol=0.900mol�����ݻ��������ܶ�=
mol=0.900mol�����ݻ��������ܶ�= =
=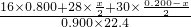 =0.780��
=0.780��
���x=0.0800mol��
����C2H4������ٷֺ���=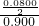 ��100%=4.44%��
��100%=4.44%��
��C2H4������ٷֺ���Ϊ4.44%��
��������1�����ݻ�����������ٷ���ɼ�Ϊ���ʵ����İٷ�������������������ù�ʽ�������ܶ�=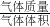 �����㣻
�����㣻
��2���������������ٷֺ������������ʵ����ٷֺ������лش���㣮
������������Ҫ����ѧ��������ٷֺ����������ʵ����ٷֺ��������⣬Ҫ��ѧ���߱����������м����������
���������ܶ�=
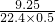 =0.826��g/l����
=0.826��g/l������0.500Ħ�û�����������Ϊ9.25g�ͱ�״���µ��ܶ�Ϊ0.826g/l��
��2���跴Ӧ֮ǰ��������ʵ���Ϊ1mol��������xmolת��ΪC2H4��������0.5xmolC2H4����0.5��0.200-x��mol���飬
��Ӧ��������������ʵ���=0.800mol+
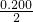 mol=0.900mol�����ݻ��������ܶ�=
mol=0.900mol�����ݻ��������ܶ�= =
=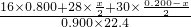 =0.780��
=0.780�����x=0.0800mol��
����C2H4������ٷֺ���=
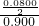 ��100%=4.44%��
��100%=4.44%����C2H4������ٷֺ���Ϊ4.44%��
��������1�����ݻ�����������ٷ���ɼ�Ϊ���ʵ����İٷ�������������������ù�ʽ�������ܶ�=
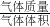 �����㣻
�����㣻��2���������������ٷֺ������������ʵ����ٷֺ������лش���㣮
������������Ҫ����ѧ��������ٷֺ����������ʵ����ٷֺ��������⣬Ҫ��ѧ���߱����������м����������

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ

 �ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��
�ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��