��Ŀ����
��ȡ����淋�����ͼ����ͼ��ʾ��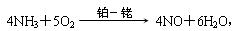
�ش��������⡣
��1���ϳɰ���ԭ��ͨ�������Ժδ���_______________________��
��2����֪N2��g����3H2��g��![]() 2NH3��g������H����92 kJ������ͣ�
2NH3��g������H����92 kJ������ͣ�
Ϊ��Ч��߰��IJ��ʣ�ʵ���������˲�ȡ��Щ��ʩ��_______________________��
��3��д�����������Ļ�ѧ����ʽ������Ͻ����к����ã�Ϊʲô����Ͻ���δԤ��Ҳ�ᷢ�ȣ�
��4����������Ĺ����г������һЩ�������������������ǶԴ�������Ⱦ��д����Ӧ�Ļ�ѧ����ʽ��
��5���ٳ�����淋�������Ҫ��;�������Ϊʲô����;���ִ����dz���Ҫ��
��6����һ���¶Ⱥ�ѹǿ���ܱ������У���ƽ����Է�������Ϊ8.5��H2��N2��ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������ƽ����Է�������Ϊ10����ʱN2��ת����Ϊ________��ƽ����������NH3���������Ϊ________��
��7������Ϊ���᳧��ѡַ������������顣
��1��N2ȡ���ڿ�����H2��������ˮú��
��2���ɲ�ȡ��ѹ���ʵ��¶Ⱥ�ʹ������ý�ȴ�ʩ
��3��![]() ����Ͻ���������á���Ϊ���Ĵ�������Ӧ�Ƿ��ȷ�Ӧ���ɱ�֤����Ͻ����ﵽһ���¶Ȳ���Ԥ�ȡ�
����Ͻ���������á���Ϊ���Ĵ�������Ӧ�Ƿ��ȷ�Ӧ���ɱ�֤����Ͻ����ﵽһ���¶Ȳ���Ԥ�ȡ�
��4�����ü�Һ���յ�������� NO��NO2��2NaOH====2NaNO2��H2O
��5��NH4NO3���������ʺ�ըҩ��ǰ�߿ɴ�ʹֲ������������ũ������������߿����ھ��¡�����������ըҩ���ʸ����ʶ��ִ����ķ�չ���ƽ�������ش�
��6��30% 17.6%
��7����ַ��ѡ��Ӧ�߱���������������ԭ�ϺͲ�Ʒ���䷽�㡢����������Դ���㡢�������ڴ������ؼ۽ϱ��˵ȡ�

 ��У����ϵ�д�
��У����ϵ�д�