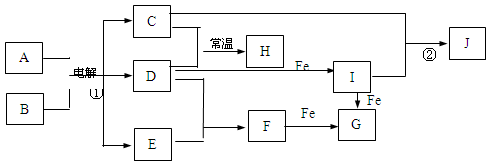
��Ŀ����
A��B��C��D��E��F�������ʵ���ɫ��Ӧ��Ϊ��ɫ�� A��B��C��D�����ᷴӦ������E������B�����ɡ��ֿ�ȼ�����壻��C��D�����ɡ�����ɫ��ζ������H����������ʹ�����ʯ��ˮ����ǡ�D��A�ɷ�Ӧ����C��F��HҲ�ɷ�Ӧ����C����������ɫ��ζ���塣
��ش��������⣺
��1��д��B��C�Ļ�ѧʽ��B��___________________; C��___________________;
��2��д��F��H2O��Ӧ�Ļ�ѧ����ʽ��__________________________________;
��3��д�����з�Ӧ�����ӷ���ʽ
��D��Һ+���_____________________________________________________;
��D��Һ+A��Һ��___________________________________________________;
��1��Na�� Na2CO3��2��2 Na2O2+2H2O=2NaOH+O2����3����HCO3-+H+=CO2��+H2O
��HCO3-+ OH-=CO32-��+H2O
����

��ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£�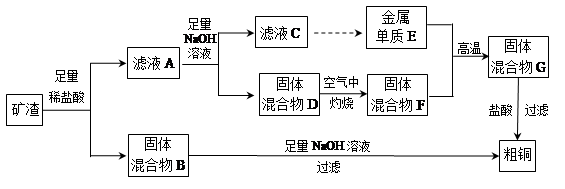
��֪��Cu2O+2H+�TCu+Cu2++H2O
��1����������B������������Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��2����ҺA����Ԫ�صĴ�����ʽֻ��ΪFe2+��������
���漰�����ӷ���ʽΪ ��
��������дCu2O������ķ�Ӧ����������ҺA��Fe2+���Լ�Ϊ �����Լ����ƣ���
��3������ҺC�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ __ ������ţ���
| A������������Һ | B��������Һ | C����ˮ | D��������̼ |
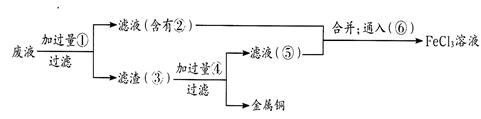
��ҵ�����÷���м�����������������������ȣ�������ʽ������[Fe(OH)SO4]�Ĺ����������£�
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�ش��������⣺
��1����ӦI��Һ�д��ڵĽ�����������__________________��
��2������NaHCO3��Ŀ���ǵ���pH��ʹ��Һ�е�______���Fe3+������Fe2+����A13+�����������ù��������С����衱��������_____________��
��3����ӦII�����ӷ���ʽΪ__________����ʵ�������У���ӦII��ͬʱͨ��O2�Լ���NaNO2��������O2��NaNO2�ڷ�Ӧ�о���Ϊ________�������뷴Ӧ��O2��11.2L(��״��)�����൱�ڽ�ԼNaNO2���ʵ���Ϊ__________��
��4����ʽ����������ˮ�������[Fe(OH)]2�����ӣ��ɲ���ˮ������[Fe2(OH)4]2���ۺ����ӡ���ˮ�ⷴӦ�����ӷ���ʽΪ__________________________��
�Ȼ�ͭ��һ�ֹ㷺�����������ϡ���ľ�ķ������Ļ�����Ʒ��ij�о���ѧϰС���ô�ͭ��������Fe�������������Ʊ��Ȼ�ͭ���塣
��1������A��ϡ�����ܽ������ˮ�ܽ��ԭ����________��
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ�X��ѡ�������Լ��е�____������ţ���
| A��NaOH | B��NH3.H2O | C��CuO | D��Cu(OH)2E. CuSO4 |
��4��ʵ���Ҳ�������ͼ��ʾװ�ã���ʹ��ͭ��Cl2��Ӧת��Ϊ����A�����ּ��������ͼг�װ������ȥ����
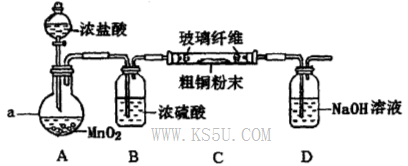
�ٸ�װ��������a��������____�����з�����Ӧ�����ӷ���ʽ��____________��
����ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊ�Ƿ��Ҫ��____________����ǡ�����
�۸�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ��_______________________��