��Ŀ����
�Ȼ�ͭ��һ�ֹ㷺�����������ϡ���ľ�ķ������Ļ�����Ʒ��ij�о���ѧϰС���ô�ͭ��������Fe�������������Ʊ��Ȼ�ͭ���塣
��1������A��ϡ�����ܽ������ˮ�ܽ��ԭ����________��
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ�X��ѡ�������Լ��е�____������ţ���
| A��NaOH | B��NH3.H2O | C��CuO | D��Cu(OH)2E. CuSO4 |
��4��ʵ���Ҳ�������ͼ��ʾװ�ã���ʹ��ͭ��Cl2��Ӧת��Ϊ����A�����ּ��������ͼг�װ������ȥ����
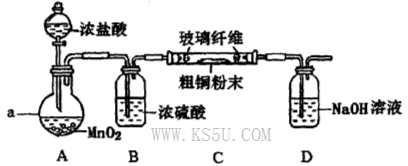
�ٸ�װ��������a��������____�����з�����Ӧ�����ӷ���ʽ��____________��
����ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊ�Ƿ��Ҫ��____________����ǡ�����
�۸�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ��_______________________��
��1������Cu2����Fe3�������ӷ���ˮ�ⷴӦ ��2��c d ��3������Ũ������ȴ�ᾧ
��4���� Բ����ƿ MnO2 + 4H++2Cl�� Mn2++ Cl2�� + 2H2O
Mn2++ Cl2�� + 2H2O
�� �� �� ��װ��C��D֮������һ��������װ��
���������������1����ͭ�к���Cu��Fe����������Ӧʱ��ʱCuCl2��FeCl3�����ڶ��߶���ǿ�������Σ�������ˮ�ⷴӦ����Cu(OH)2��Fe(OH)3��Ϊ������Cu2����Fe3�������ӷ���ˮ�ⷴӦͨ����ϡ�������ܽ⡣
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ��������������µ��������ӡ�������Ŀ���������ʣ���ѡ��CuO��Cu(OH)2��ѡ��ΪCD
��3������ȥFe(OH)3�����ĺ���CuCl2��Һ��һϵ�в����ɵ��Ȼ�ͭ���塣CuCl2��ǿ�������Σ�ˮ�����Cu(OH)2��HCl�������лӷ��ԣ����ӷ��ݳ����õ��Ĺ�����Cu(OH)2�����������ܽ�����¶ȵ�Ӱ��仯�ϴ����Կɲ��õIJ����ij�������Ϊ����Ũ������ȴ�ᾧ�����ˡ���Ȼ�����4��ʵ������ȡCl2����������ƿ����Ũ������������̹��ȵķ�����ȡ�ġ���Ӧ����������ʽΪ��MnO2 + 4H++2Cl�� Mn2++ Cl2�� + 2H2O������ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊû�б�Ҫ����ΪCu��HCl���ᷢ����Ӧ��ֻ��Cl2������Ӧ��������Cl2������NaOH������Ӧ�����µ������е�ѹǿ��С����ʱ�ձ��е���Һ������������װ�ã��ʸ�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ����װ��C��D֮������һ��������װ�á�
Mn2++ Cl2�� + 2H2O������ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊû�б�Ҫ����ΪCu��HCl���ᷢ����Ӧ��ֻ��Cl2������Ӧ��������Cl2������NaOH������Ӧ�����µ������е�ѹǿ��С����ʱ�ձ��е���Һ������������װ�ã��ʸ�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ����װ��C��D֮������һ��������װ�á�
���㣺����������Ʊ��������ķ��롢�ε�ˮ���֪ʶ��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д��ú���A12O3��SiO2������FeO·xFe2O3�������Ʊ�A12(SO4)3·18H2O����������������(���ֲ�����������):
��.�������м������ϡH2SO4������;
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ;
��.����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
��.Ũ�����ᾧ�����룬�õ���Ʒ��
H2SO4�ܽ�A12O3�����ӷ���ʽ��
��KMnO4 ����Fe2+�����ӷ���ʽ���������� MnO4-+��Fe2++�� =
MnO4-+��Fe2++�� = Mn2++��Fe3+ +��
Mn2++��Fe3+ +��
��ʽ���������� ,���������� ��
��3����֪�������������������pH ks5u
| | Al��OH��3 | Fe��OH��2 | Fe��OH��3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�ģ� ��
��֪:һ�������£�MnO4 - ����Mn2+��Ӧ����MnO2��
�� �� �� �ij����м���ŨHCI�����ȣ���˵�������д���MnO2�������� ��
�ڢ� �м���MnSO4��Ŀ���� ��
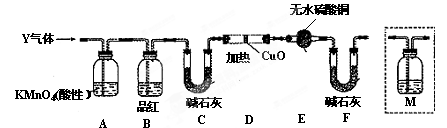
Fe2O3���й㷺����;����ͬѧ�Ķ��й����ϵ�֪���ڸ���������FeCO3���Եõ�Fe2O3��Ϊ�˽�һ����֤�˽��ۣ�����������ʵ�飺
| ʵ�鲽�� | ʵ����� |
| �� | ȡһ��������FeCO3�������������У������������������ټ��ᣬ��ȴ������ |
| �� | ȡ����ʵ�鲽��I���ù������һ�ྻ���Թ��У���������ϡ�����ܽ� |
| �� | ��ʵ�鲽���������Һ�еμ�KSCN��Һ����Һ��� |
�ɴ˼�ͬѧ�ó����ۣ�4FeCO3��O2
 2Fe2O3��4CO2
2Fe2O3��4CO2(1)д��ʵ�鲽����з�����Ӧ�����ӷ���ʽ__________________________________��
(2)��ͬѧ����˲�ͬ�Ŀ��������ղ��������Fe3O4����ΪFe3O4Ҳ�����������ᣬ��������Һ��Ҳ����Fe3����������ͬѧ�Լ�ͬѧ��ʵ�鲽�������˲���Ľ�������ʵ�鲽���������Һ���Ƿ���Fe2��������Ҫѡ����Լ���____________(�����)��
a����ˮ b����ˮ��KSCN��Һ c��K3[Fe(CN)6](���軯����Һ)
(3)��ͬѧ��Ϊ��ʹ�õ�����ͬѧԤ�ڵ�ʵ������Ҳ����ȷ�����ղ���ijɷ֡�����Ϊ��ͬѧ�ִ˿�����������____________��
(4)��ͬѧ��һ���������ϵ�֪������FeCO3�IJ����е�ȷ���У�2����Ԫ�ء��������������һ����FeCO3��ȡFe2O3�ķ���������FeCO3�����μ����Լ���ϡ���ᡢ________ (���Լ�����)�Ͱ�ˮ����_________(���������)�����գ����ɵõ�Fe2O3��
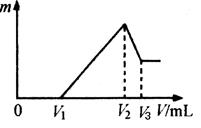
(5)��ҵ����������ԭ�ζ�������������FeCO3������������ͨ��������Ʒ��������ʹ�ζ�ʱ����KMnO4��Һ���Ϊc mL����Ӧ��������FeCO3����������Ϊc%�����Լ��㡣ijͬѧȡ��FeCO3 c%��������a g��������ϡ�����ܽ������0.0 200 mol��L��1������KMnO4��Һ�ζ�(KMnO4����ԭ��Mn2��)����������KMnO4��Һc mL���ٶ���ʯ����������ԭ�����ʣ�����ȡ�����������a��__________g��(FeCO3��Ħ������Ϊ116 g��mol��1)

 ��Ԥ��ʵ������Ӧ�� ��
��Ԥ��ʵ������Ӧ�� ��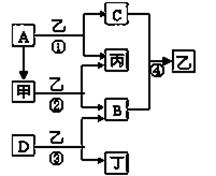
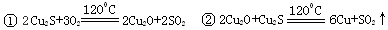



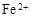 ��
�� �⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
�⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��