��Ŀ����
��ͼΪijЩ��������֮���ת����ϵ����֪��A��B��I�к�����ͬ���������Ҷ���XY2�ͻ������I��ʵ���ҳ��õĸ������CΪֱ���ͷ��ӣ�E��FΪ�ǽ������嵥�ʣ�
�밴Ҫ����գ�

��1����B�ĵ���ʽ��______����K�Ľṹʽ��______��
��2��D��G��Ӧ�Ļ�ѧ����ʽ��______��
��3����֪C��ȼ������1300kJ/mol����ʾC��ȼ���ȵ��Ȼ�ѧ����ʽ��______��
��4����G����ˮ�����Һ�������������ҺG�����������ӵIJ���������______��
��5��������0.1mol/L��J��Һ��c��H+��/c��OH-��=1��10-8�����������������______��
A������Һ��pH=11��
B������Һ�е����ʵ������������Ũ��0.1mol/L
C������Һ��ˮ�������c��H+����c��OH-���˻�Ϊ1��10-22
D��pH=3��������ҺV1 L���0.1mol/L��J��ҺV2 L��ϣ��������ҺpH=7����V1��V2
E����������Һ��ˮϡ��100����pHֵΪ9��
��6������F�ڹ�ҵ������Ҫ����;��______�����������֣�
�⣺�������⡰CΪֱ���ͷ��ӡ����ӿ�ͼ���Կ���C��A��ˮ��Ӧ���ɵģ����Գ���ȷ��C����Ȳ����AΪ̼���ƣ�D���������ƣ���ͼ�е�G�����̣�����ѧѧ���ľ����Ȼ�泥�����������Ϊ����㣬��֪�������ʷֱ�Ϊ��B��CaO2��E��������F��������G�����ܼ�������������������ͭ�ȣ�H�Ǵ�����ƣ�I���Ȼ��ơ�J���Ȼ����ȡ�K�Ǵ����ᣬN��HCl��
��1���ٹ������������ӻ��������ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
�ڴ������ǹ��ۻ������Ԫ�ء���Ԫ���Լ���Ԫ�ؾ��ﵽ���ȶ��ṹ���ṹʽΪ��H-O-Cl���ʴ�Ϊ��H-O-Cl��
��2��ʵ�����������Ȼ�狀��������Ʒ�Ӧ����ȡ��������Ӧԭ��Ϊ��2NH4Cl+Ca��OH��2 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��3��ȼ����ʵָ1ml��ȼ��ȼ���������ȶ����������ͷų���������������Ȳȼ�յķ���ʽΪ��C2H2��g��+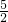 O2��g��=2CO2��g��+H2O��l����H=-1300kJ/mol��
O2��g��=2CO2��g��+H2O��l����H=-1300kJ/mol��
�ʴ�Ϊ��C2H2��g��+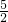 O2��g��=2CO2��g��+H2O��l����H=-1300kJ/mol��
O2��g��=2CO2��g��+H2O��l����H=-1300kJ/mol��
��4��笠����ӵļ���ԭ���ǣ�笠����Ӻ�ǿ���ڼ��ȵ�������������ʹʪ��ĺ�ɫʯ����ֽ�����İ�����
�ʴ�Ϊ��ȡ�������Ȼ����Һ���Թ��У���������������������Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ�����ɫ������NH4+��
��5��A��0.1mol/L���Ȼ�����Һ�У�c��H+��/c��OH-��=1��10-8��c��H+��?c��OH-��=10-14������c��H+��=10-11mol/L��������Һ��pH=11����A��ȷ��
B��0.1mol/L�İ�ˮ��Һ�У���Ϊһˮ�ϰ���������ʣ�����ȫ���룬笠����ӵ�Ũ��С��0.1mol/L����B����
C�������£�����Һ��ˮ�������c��H+����c��OH-���˻�Ϊ1��10-14���¶Ȳ��䣬��ֵ���䣬��C����
D��pH=3��������ҺV1 L���0.1mol/L�İ�ˮ��ҺV2 L��ϣ��������ҺpH=7������Ҫ���������V1��V2����D��ȷ��
E��һˮ�ϰ���������ʣ���ˮϡ��100����pHֵ����9����E����
��ѡBCE��
��6�������Ĺ�ҵ��;����Ư�ۣ������ᣨ����Ư��Һ�ȣ����ʴ�Ϊ����Ư�ۣ������ᣨ��Ư��Һ�ȣ���
����������ͼ�ƶ��⣬Ҫ���ڴ���ɺͿ�ͼ��Ѱ��ͻ�ƿڣ�����ıȽ�ֱ�ӵ�ͻ�ƿ���������һ������С�CΪֱ���ͷ��ӡ����ӿ�ͼ���Կ���C��A��ˮ��Ӧ���ɵģ����Գ���ȷ��C����Ȳ����AΪ̼���ƣ�D���������ƣ���ͼ�е�G�����̣�����ѧѧ���ľ����Ȼ�泥�����������Ϊ����㣬��֪�������ʷֱ�Ϊ��B��CaO2��E��������F��������G�����ܼ�������������������ͭ�ȣ�H�Ǵ�����ƣ�I���Ȼ��ơ�J���Ȼ����ȡ�K�Ǵ����ᣬN��HCl��
������������һ����ͼ�ƶ��⣬����֪ʶ��Ϲ㣬Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�
��1���ٹ������������ӻ��������ʽΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
���ڴ������ǹ��ۻ������Ԫ�ء���Ԫ���Լ���Ԫ�ؾ��ﵽ���ȶ��ṹ���ṹʽΪ��H-O-Cl���ʴ�Ϊ��H-O-Cl��
��2��ʵ�����������Ȼ�狀��������Ʒ�Ӧ����ȡ��������Ӧԭ��Ϊ��2NH4Cl+Ca��OH��2
 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca��OH��2
 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O����3��ȼ����ʵָ1ml��ȼ��ȼ���������ȶ����������ͷų���������������Ȳȼ�յķ���ʽΪ��C2H2��g��+
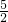 O2��g��=2CO2��g��+H2O��l����H=-1300kJ/mol��
O2��g��=2CO2��g��+H2O��l����H=-1300kJ/mol���ʴ�Ϊ��C2H2��g��+
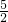 O2��g��=2CO2��g��+H2O��l����H=-1300kJ/mol��
O2��g��=2CO2��g��+H2O��l����H=-1300kJ/mol����4��笠����ӵļ���ԭ���ǣ�笠����Ӻ�ǿ���ڼ��ȵ�������������ʹʪ��ĺ�ɫʯ����ֽ�����İ�����
�ʴ�Ϊ��ȡ�������Ȼ����Һ���Թ��У���������������������Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ�����ɫ������NH4+��
��5��A��0.1mol/L���Ȼ�����Һ�У�c��H+��/c��OH-��=1��10-8��c��H+��?c��OH-��=10-14������c��H+��=10-11mol/L��������Һ��pH=11����A��ȷ��
B��0.1mol/L�İ�ˮ��Һ�У���Ϊһˮ�ϰ���������ʣ�����ȫ���룬笠����ӵ�Ũ��С��0.1mol/L����B����
C�������£�����Һ��ˮ�������c��H+����c��OH-���˻�Ϊ1��10-14���¶Ȳ��䣬��ֵ���䣬��C����
D��pH=3��������ҺV1 L���0.1mol/L�İ�ˮ��ҺV2 L��ϣ��������ҺpH=7������Ҫ���������V1��V2����D��ȷ��
E��һˮ�ϰ���������ʣ���ˮϡ��100����pHֵ����9����E����
��ѡBCE��
��6�������Ĺ�ҵ��;����Ư�ۣ������ᣨ����Ư��Һ�ȣ����ʴ�Ϊ����Ư�ۣ������ᣨ��Ư��Һ�ȣ���
����������ͼ�ƶ��⣬Ҫ���ڴ���ɺͿ�ͼ��Ѱ��ͻ�ƿڣ�����ıȽ�ֱ�ӵ�ͻ�ƿ���������һ������С�CΪֱ���ͷ��ӡ����ӿ�ͼ���Կ���C��A��ˮ��Ӧ���ɵģ����Գ���ȷ��C����Ȳ����AΪ̼���ƣ�D���������ƣ���ͼ�е�G�����̣�����ѧѧ���ľ����Ȼ�泥�����������Ϊ����㣬��֪�������ʷֱ�Ϊ��B��CaO2��E��������F��������G�����ܼ�������������������ͭ�ȣ�H�Ǵ�����ƣ�I���Ȼ��ơ�J���Ȼ����ȡ�K�Ǵ����ᣬN��HCl��
������������һ����ͼ�ƶ��⣬����֪ʶ��Ϲ㣬Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�
�����Ŀ







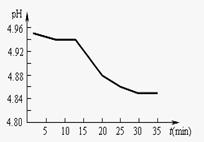 ��2��SO2���ŷ�������������Ҫ���ء�ij��������
��2��SO2���ŷ�������������Ҫ���ء�ij��������