��Ŀ����
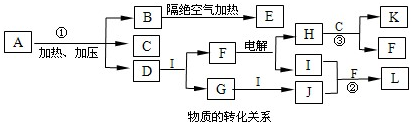
��ͼ��ÿһ�����ʾ�йص�һ�ַ�Ӧ������������B��DΪ��ɫ���壮

��1����ͼ��A����Ϊ
 ��D�Ŀռ乹��Ϊ
��D�Ŀռ乹��Ϊ
��2����Ӧ�ٵĻ�ѧ����ʽΪ
��3����ӦD��F��ÿ����1gҺ̬C������akJ����������D��F���Ȼ�ѧ����ʽΪ��

��1����ͼ��A����Ϊ
̼���
̼���
��̼�����
̼�����
��B�ĵ���ʽ

����
����
��G����ɫ����ɫ
����ɫ
����2����Ӧ�ٵĻ�ѧ����ʽΪ
2CO2+2Na2O2�T2Na2CO3+O2
2CO2+2Na2O2�T2Na2CO3+O2
����3����ӦD��F��ÿ����1gҺ̬C������akJ����������D��F���Ȼ�ѧ����ʽΪ��
4NH3��g��+5O2��g���T4NO��g��+6H2O��l����H=+108akJ?mol-1
4NH3��g��+5O2��g���T4NO��g��+6H2O��l����H=+108akJ?mol-1
��������A���������ᷴӦ��������NaOH��Ӧ�����������壬�����ڼ��������·ֽ⣬�����ɵIJ�������������Ʒ�Ӧ����AӦΪ̼��炙�̼����泥�BΪCO2��CΪH2O��DΪNH3��EΪO2��FΪNO��GΪNO2��HΪHNO3��������ʵ����ʿɽ����⣮
����⣺A���������ᷴӦ��������NaOH��Ӧ�����������壬�����ڼ��������·ֽ⣬�����ɵIJ�������������Ʒ�Ӧ����AӦΪ̼��炙�̼����泥�BΪCO2��CΪH2O��DΪNH3��EΪO2��FΪNO��GΪNO2��HΪHNO3��
��1��AΪ̼��炙�̼����泥�BΪCO2������ʽΪ ��DΪNH3��Ϊ�����Σ�GΪNO2��Ϊ����ɫ���壬
��DΪNH3��Ϊ�����Σ�GΪNO2��Ϊ����ɫ���壬
�ʴ�Ϊ��̼��泥�̼����泥� �������Σ�����ɫ��
�������Σ�����ɫ��
��2����Ӧ��ΪNa2O2��CO2�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ2CO2+2Na2O2�T2Na2CO3+O2��
�ʴ�Ϊ��2CO2+2Na2O2=2Na2CO3+O2��
��3��n��H2O��=
mol������akJ��������������6molH2O����6��18akJ=108akJ����Ӧ���Ȼ�ѧ����ʽΪ4NH3��g��+5O2��g���T4NO��g��+6H2O��l����H=+108akJ?mol-1��
�ʴ�Ϊ��4NH3��g��+5O2��g���T4NO��g��+6H2O��l����H=+108akJ?mol-1��
��1��AΪ̼��炙�̼����泥�BΪCO2������ʽΪ
 ��DΪNH3��Ϊ�����Σ�GΪNO2��Ϊ����ɫ���壬
��DΪNH3��Ϊ�����Σ�GΪNO2��Ϊ����ɫ���壬�ʴ�Ϊ��̼��泥�̼����泥�
 �������Σ�����ɫ��
�������Σ�����ɫ����2����Ӧ��ΪNa2O2��CO2�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ2CO2+2Na2O2�T2Na2CO3+O2��
�ʴ�Ϊ��2CO2+2Na2O2=2Na2CO3+O2��
��3��n��H2O��=
| 1 |
| 18 |
�ʴ�Ϊ��4NH3��g��+5O2��g���T4NO��g��+6H2O��l����H=+108akJ?mol-1��
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע���A�������ƶϳ�A����Ϊ̼��炙�̼����泥����ݳ���Ԫ�ػ���������ʿ������Ƶķ������

��ϰ��ϵ�д�
 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ

 NH4++NH2-
NH4++NH2-


