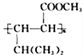��Ŀ����
����Ŀ����Һ�еĻ�ѧ��Ӧ��������ӷ�Ӧ������Ҫ��ش��������⣺
��1���μ��(���϶�Na2CO3��NaCl)������ֲ���������������ӷ���ʽ��ʾ�μ�سʼ��Ե�ԭ��____________________________________��
��2����֪ˮ����ƽ��2H2O![]() H3O����OH������ˮ�м���NaHSO4���壬ˮ�ĵ���ƽ��________�ƶ���������Һ��________�ԡ�
H3O����OH������ˮ�м���NaHSO4���壬ˮ�ĵ���ƽ��________�ƶ���������Һ��________�ԡ�
��3����ȡpH���������ȵ�NaOH��Һ�Ͱ�ˮ�ֱ��ˮϡ��m����n����ϡ�ͺ�pH����ȣ���m________ n(���������������)��
��4�������£���pH��6��CH3COOH��CH3COONa�Ļ����Һ�У���ˮ���������c(OH��)��________mol��L��1��
��5����֪�������£�NH3��H2O�ĵ���ƽ�ⳣ��Kb��1.75��10��5��H2CO3�ĵ���ƽ�ⳣ��Ka1��Ka2�ֱ�Ϊ4.4��10��7��5.6��10��11, ��������Kb��__________(����Ka1�� ���� Ka2��)����֪NH4HCO3��Һ���������________�ԡ�
���𰸡� CO![]() ��H2O
��H2O![]() HCO
HCO![]() ��OH�� ���� �� �� 1��10��8 Ka1 ��
��OH�� ���� �� �� 1��10��8 Ka1 ��
����������1��̼����ˮ�⣬��Һ�Լ��ԣ�����μ�سʼ��Ե�ԭ����CO32����H2O![]() HCO3����OH������2����ˮ�м���NaHSO4���壬������Ũ������ˮ�ĵ���ƽ�������ƶ���������Һ����������3��ϡ�����дٽ�һˮ�ϰ��ĵ��룬�����������ʵ������ӣ�����Ҫʹϡ�͵ĺ��pH��ȣ���ˮϡ�͵ı�����m��n����4�������£���pH��6��CH3COOH��CH3COONa�Ļ����Һ�д���ĵ���̶ȴ��ڴ������ˮ��̶��������ˮ���������c(OH��)��
HCO3����OH������2����ˮ�м���NaHSO4���壬������Ũ������ˮ�ĵ���ƽ�������ƶ���������Һ����������3��ϡ�����дٽ�һˮ�ϰ��ĵ��룬�����������ʵ������ӣ�����Ҫʹϡ�͵ĺ��pH��ȣ���ˮϡ�͵ı�����m��n����4�������£���pH��6��CH3COOH��CH3COONa�Ļ����Һ�д���ĵ���̶ȴ��ڴ������ˮ��̶��������ˮ���������c(OH��)��![]() ����5������Kb��Ka1������̼�������ˮ��̶ȴ���笠���ˮ��̶ȣ���NH4HCO3��Һ�Լ��ԡ�
����5������Kb��Ka1������̼�������ˮ��̶ȴ���笠���ˮ��̶ȣ���NH4HCO3��Һ�Լ��ԡ�

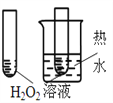
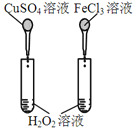
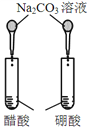
����Ŀ����ͼ��ʾ��ʵ�飬���ܴﵽʵ��Ŀ�ĵ��ǣ���ѡ���жԱ���Һ��Ũ���������ͬ��( )
ʵ�鷽�� |
|
|
|
|
Ŀ�� | A����֤�����¶ȿɼӿ�H2O2�ֽ� | B����֤����Ӧ��Ũ�ȶ�ƽ���Ӱ�� | C���Ƚ�Cu2+��Fe3+��H2O2�ֽ����ʵ�Ӱ�� | D���Ƚ���������ǿ�� |
A. A B. B C. C D. D