��Ŀ����
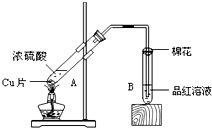
ijУ��ѧС���ͬѧ����ͼװ�ö�ͭ��Ũ������һ�������µķ�Ӧ����ʵ��̽������ش�

��1��д��A�Թ��з�����Ӧ�Ļ�ѧ����ʽ______������1Ħͭ��ȫ��Ӧ����ԭ��H2SO4�����ʵ���Ϊ______��
��2������д���пհף�
| B������� | �� | �� | �� |
| ��պ�Լ� | ���з�̪��NaOH��Һ | Ʒ����Һ | ���ۺ͵�ˮ���Һ |
| ���� | ______ | ______ | ______ |
| ����SO2������ | ______ | ______ | ______ |
��4����C�����ڻ�������β����װ��ͼ��������Լ����ƣ�
�⣺��1��A�Թ��з�����Ӧ��ͭ��Ũ����ķ�Ӧ����Ӧ����ʽ�ǣ�Cu+2H2SO4 ��Ũ�� CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
���ݷ�Ӧ����ʽ��1Ħͭ��ȫ��Ӧ����ԭ��Ũ������1mol��
�ʴ�Ϊ��Cu+2H2SO4 ��Ũ�� CuSO4+SO2��+2H2O��1mol��
CuSO4+SO2��+2H2O��1mol��
��2���ٶ�������ͨ�����з�̪��NaOH��Һ����Һ��ɫ��ʧ�������˶�����������ԣ�
�ʴ�Ϊ����Һ��ɫ��ʧ�����ԣ�
�ڶ�������ͨ��Ʒ����Һ��Ʒ����ɫ�������˶��������Ư���ԣ�
�ʴ�Ϊ��Ʒ����ɫ��Ư���ԣ�
�۶�������ͨ�����ۺ͵�ˮ���Һ������������������ɫ��ɫ�������˶�������Ļ�ԭ�ԣ�
�ʴ�Ϊ����ɫ��ɫ����ԭ�ԣ�
��3����������͵ⷴӦ�����ӷ���ʽ�ǣ�SO2+I2+2H2O=4H++2I-+SO42-��
�ʴ�Ϊ��SO2+I2+2H2O=4H++2I-+SO42-��
��4��ʹ������������Һ���ն���Ķ������ʴ�Ϊ�� ��
��
��������1��ͭ��Ũ���ᷴӦ���ɶ�����������ͭ��ˮ��
��2��������պ�еIJ�ͬ�Լ��жϷ���������Ͷ�����������ʣ�
��3������������������������ӣ�
��4������ʹ������������Һ����δ��Ӧ�Ķ�������
���������⿼����ͭ��Ũ����ķ�Ӧ���漰�˷�Ӧ����ʽ��д��β��������֪ʶ���Ѷ��еȣ�
 CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O�����ݷ�Ӧ����ʽ��1Ħͭ��ȫ��Ӧ����ԭ��Ũ������1mol��
�ʴ�Ϊ��Cu+2H2SO4 ��Ũ��
 CuSO4+SO2��+2H2O��1mol��
CuSO4+SO2��+2H2O��1mol����2���ٶ�������ͨ�����з�̪��NaOH��Һ����Һ��ɫ��ʧ�������˶�����������ԣ�
�ʴ�Ϊ����Һ��ɫ��ʧ�����ԣ�
�ڶ�������ͨ��Ʒ����Һ��Ʒ����ɫ�������˶��������Ư���ԣ�
�ʴ�Ϊ��Ʒ����ɫ��Ư���ԣ�
�۶�������ͨ�����ۺ͵�ˮ���Һ������������������ɫ��ɫ�������˶�������Ļ�ԭ�ԣ�
�ʴ�Ϊ����ɫ��ɫ����ԭ�ԣ�
��3����������͵ⷴӦ�����ӷ���ʽ�ǣ�SO2+I2+2H2O=4H++2I-+SO42-��
�ʴ�Ϊ��SO2+I2+2H2O=4H++2I-+SO42-��
��4��ʹ������������Һ���ն���Ķ������ʴ�Ϊ��
 ��
����������1��ͭ��Ũ���ᷴӦ���ɶ�����������ͭ��ˮ��
��2��������պ�еIJ�ͬ�Լ��жϷ���������Ͷ�����������ʣ�
��3������������������������ӣ�
��4������ʹ������������Һ����δ��Ӧ�Ķ�������
���������⿼����ͭ��Ũ����ķ�Ӧ���漰�˷�Ӧ����ʽ��д��β��������֪ʶ���Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 ijУ��ѧС���ͬѧ����ͼװ�ö�̿��Ũ����ķ�Ӧ�IJ��������֤����ش��������⣺
ijУ��ѧС���ͬѧ����ͼװ�ö�̿��Ũ����ķ�Ӧ�IJ��������֤����ش��������⣺