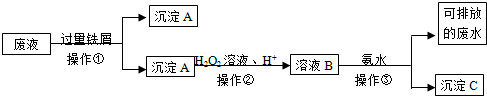
��Ŀ����
��һ�������������Na2CO3��Na2SO4��CuSO4��CaCl2��NaCl��϶��ɣ�Ϊ�������ǵ���ɣ���������ʵ�飺
��1����������������ˮ�������õ���ɫ����Һ��
��2��������Һ�еμ����ᱵ��Һ���а�ɫ�������ɣ�
��3�������ɳ������Թ��м�����ϡ���ᣬ����ȫ���ܽ⣬������ɫ���������
���жϣ����������п϶��У������ʽ�� ���϶�û�� ��
�ڣ�2����ʵ�鷴Ӧ�����ӷ���ʽ��
�ڣ�3����ʵ�鷴Ӧ�����ӷ���ʽ�� ��
��1����������������ˮ�������õ���ɫ����Һ��
��2��������Һ�еμ����ᱵ��Һ���а�ɫ�������ɣ�
��3�������ɳ������Թ��м�����ϡ���ᣬ����ȫ���ܽ⣬������ɫ���������
���жϣ����������п϶��У������ʽ��
�ڣ�2����ʵ�鷴Ӧ�����ӷ���ʽ��
�ڣ�3����ʵ�鷴Ӧ�����ӷ���ʽ��
���㣺����δ֪��ļ���
ר�⣺���ʼ��������
�������ɣ�1�����������ˮ����������ɫ����Һ����һ������CuSO4����Na2CO3��Na2SO4��CaCl2����ͬʱ���ڣ�
�ɣ�2������Һ�еμ����ᱵ��Һ���а�ɫ�������ɣ����ɫ����Ϊ̼�ᱵ�����ᱵ���ٽ�ϣ�3����֪��ɫ������ȫ�ܽ��������У����ɫ����Ϊ̼�ᱵ���Դ������
�ɣ�2������Һ�еμ����ᱵ��Һ���а�ɫ�������ɣ����ɫ����Ϊ̼�ᱵ�����ᱵ���ٽ�ϣ�3����֪��ɫ������ȫ�ܽ��������У����ɫ����Ϊ̼�ᱵ���Դ������
���
�⣺�ɣ�1�����������ˮ����������ɫ����Һ����һ������CuSO4����Na2CO3��Na2SO4��CaCl2����ͬʱ���ڣ�
�ɣ�2������Һ�еμ����ᱵ��Һ���а�ɫ�������ɣ����ɫ����Ϊ̼�ᱵ�����ᱵ���ٽ�ϣ�3����֪��ɫ������ȫ�ܽ��������У����ɫ����Ϊ̼�ᱵ����һ����Na2CO3����ӦΪ��Ba2++CO32-=BaCO3������һ������Na2SO4��CaCl2������ȷ���Ƿ�NaCl��
���������п϶��У�Na2CO3��һ������CuSO4��Na2SO4��CaCl2������ȷ���Ƿ�NaCl���ڣ�2����ʵ�鷴Ӧ�����ӷ���ʽ��Ba2++CO32-=BaCO3�����ڣ�3����ʵ�鷴Ӧ�����ӷ���ʽ��BaCO3+2H+=CO2��+H2O+Ba2+��
�ʴ�Ϊ��Na2CO3��CuSO4��Na2SO4��CaCl2��Ba2++CO32-=BaCO3����BaCO3+2H+=CO2��+H2O+Ba2+��
�ɣ�2������Һ�еμ����ᱵ��Һ���а�ɫ�������ɣ����ɫ����Ϊ̼�ᱵ�����ᱵ���ٽ�ϣ�3����֪��ɫ������ȫ�ܽ��������У����ɫ����Ϊ̼�ᱵ����һ����Na2CO3����ӦΪ��Ba2++CO32-=BaCO3������һ������Na2SO4��CaCl2������ȷ���Ƿ�NaCl��
���������п϶��У�Na2CO3��һ������CuSO4��Na2SO4��CaCl2������ȷ���Ƿ�NaCl���ڣ�2����ʵ�鷴Ӧ�����ӷ���ʽ��Ba2++CO32-=BaCO3�����ڣ�3����ʵ�鷴Ӧ�����ӷ���ʽ��BaCO3+2H+=CO2��+H2O+Ba2+��
�ʴ�Ϊ��Na2CO3��CuSO4��Na2SO4��CaCl2��Ba2++CO32-=BaCO3����BaCO3+2H+=CO2��+H2O+Ba2+��
���������⿼��δ֪����ƶϣ�Ϊ��Ƶ���㣬�����ڿ���ѧ�����ۺϷ����������������ʵ����ʡ������ķ�Ӧ��һ�����ƶϼ��ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
�����Ŀ
 ��һ�������£����ڷ�ӦmA��g��+nB��g��?cC��g��+dD��g����C���ʵ�Ũ�ȣ�c�����¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������
��һ�������£����ڷ�ӦmA��g��+nB��g��?cC��g��+dD��g����C���ʵ�Ũ�ȣ�c�����¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������| A����H��0��S��0 |
| B����H��0��S��0 |
| C����S��0��H��0 |
| D����H��0��S��0 |
1Lij��Һ�к��е�����������ö��Ե缫������Һ������·����3mol e-ͨ��ʱ�����Ե��ʱ��Һ����ı仯���缫������ܴ��ڵ��ܽ���������˵����ȷ���ǣ�������
| ���� | Cu2+ | Al3+ | NO | Cl- |
| ���ʵ���Ũ�ȣ�mol/L�� | 1 | 1 | a | 1 |
| A��������Һ��pH=0 |
| B��a=3 |
| C����������1.5 mol Cl2 |
| D�����������Ľ�����ͭ���� |
���������в������ڼ��ȵ��ǣ�������
| A�������� | B����Ͳ | C���ձ� | D������ |
�������л���ṹ��������ص�������ȷ���ǣ�������
| A��������������֬���������ɿ���ȡ����Ӧ |
| B�����ᡢ����������Ϊͬ���칹�� |
| C���Ҵ�����������ж������ǻ������Ƕ��ܺ��������Ʒ�Ӧ����H2O |
| D�����ࡢ��֬�͵����ʶ��ܷ���ˮ�⣬���ȼ�յIJ��ﶼ��CO2��H2O |
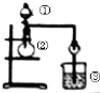
 ��1���л���X ������ͼΪ��
��1���л���X ������ͼΪ��