��Ŀ����
5��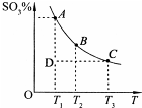 ����������������������һ�����ҵĻ���������������ҵ��������������д��ڷ�Ӧ��2SO2��g��+O2��g��?2SO3��g������Ӧ��ϵ��SO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
����������������������һ�����ҵĻ���������������ҵ��������������д��ڷ�Ӧ��2SO2��g��+O2��g��?2SO3��g������Ӧ��ϵ��SO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺��1��������ԭ�ӵĽṹʾ��ͼ

��2������˵����ȷ����bd
a�����ں��¡���ѹ������������ƽ����ϵ��ͨ�뺤����ƽ�ⲻ�ƶ�
b����D��ʱ��v����v��
c��B�㡢C�㷴Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2
d����A��ʱ������1mol SO2�ض�ͬʱ����1mol SO3��
���� ��1��������ԭ�Ӻ�����16�����ӣ�������16�����ӣ��ֱ�Ϊ2��8��6�Ų�����ԭ�ӵĽṹʾ��ͼ��
��2��a�����¡���ѹ������������ƽ����ϵ��ͨ�뺤�������Ӧ����Ӧ��������ֵ�Ũ�Ƚ��ͣ���ЧΪ����ѹǿ��ѹǿ����ƽ��������������ƶ���
b��D״̬δ��ƽ�⣬�����ϵ��SO3�İٷֺ���С��ƽ��ʱ�ģ���Ӧ������Ӧ���н���ƽ�⣻
c����ͼ��֪���¶�Խ�ߣ������ϵ��SO3�İٷֺ���ԽС��˵�������¶�ƽ�����淴Ӧ���У��ݴ��жϣ�
d����A��Ϊƽ��㣬���淴Ӧ������Ƚ����жϣ�
��� �⣺��1����ԭ�ӽṹʾ��ͼ�У�ԲȦ��ʾԭ�Ӻˣ�ԲȦ�ڵ���ֵΪ��������������Ӳ��û��߱�ʾ�������ϵ����ݱ�ʾÿ��ĵ���������ԭ�ӵ���������16��ԭ�ӽṹʾ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��a�����¡���ѹ������������ƽ����ϵ��ͨ�뺤�������Ӧ����Ӧ��������ֵ�Ũ�Ƚ��ͣ���ЧΪ����ѹǿ��ѹǿ����ƽ��������������ƶ����������ƶ����ʴ���
b��D״̬δ��ƽ�⣬�����ϵ��SO3�İٷֺ���С��ƽ��ʱ�ģ���Ӧ������Ӧ���н���ƽ�⣬����V����V��������ȷ��
c����ͼ��֪���¶�Խ�ߣ������ϵ��SO3�İٷֺ���ԽС��˵�������¶�ƽ�����淴Ӧ���У�Kֵ��С����ƽ�ⳣ��K1��K2���ʴ���
d����A��Ϊƽ��㣬���淴Ӧ������ȣ���������1mol SO2�ض�ͬʱ����1mol SO3������ȷ��
��ѡ��bd��
���� ���⿼���˻�ѧƽ�⼰��ͼ�������ѶȲ���ע�����֪ʶ�Ļ��ۣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | B�� | �Ҵ��ķ���ʽ��CH3CH2OH | ||
| C�� | CH2F2�ĵ���ʽ�� | D�� | ��ȩ�Ľṹʽ�� |
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | �� |
��1������ЩԪ���У���ѧ��������õ�Ԫ�ط���ΪAr����ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ
 ��
����2���ؿ��к������Ľ���Ԫ����Al���ߡ�������Ԫ�ص��⻯���ȶ�����ǿ������˳��ΪHCl��H2S��
��4����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����NaOH��
��5��Ԫ�آ۵�����������Һ��Ԫ�آݵ��������ﷴӦ�����ӷ���ʽΪ��OH-+Al��OH��3=AlO2-+2H2O��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
 ��
����2����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����KOH
��3���ݵĵ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2�����ݵ���������������������Һ��Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
��4�����ߵ���ͨ��������ɵĻ������У�������Ӧ�����ӷ���ΪCl2+2Br-=2Cl-+Br2��
��5���ٵĵ��ʳ�������ҵ�Ϻϳ�ij�ּ������壬д���ü������屻�����������ķ�Ӧ��ѧ����ʽ4NH3+5O2$\frac{\underline{\;\;����\;\;}}{���¸�ѹ}$4NO+6H2O��
| A�� | ��AlCl3��Һ������NaOH��Һ��������Al3++3OH-�TAl��OH��3�� | |
| B�� | ��NaOH��Һ������AlCl3��Һ��������Al3++4OH-�TAlO2-+2H2O | |
| C�� | �ڰ�ˮ�м��뼸����������Һ��Fe3++3OH-�TFe��OH��3�� | |
| D�� | ��Na2CO3��Һ�еμӼ���ϡ���CO32-+H+�THCO3- |
 A��B��X��Y��Ϊ��ѧ�εij������ʣ�����֮���ת����ϵ��ͼ��ʾ����ش��������⣺
A��B��X��Y��Ϊ��ѧ�εij������ʣ�����֮���ת����ϵ��ͼ��ʾ����ش��������⣺

 ��
�� ��
��