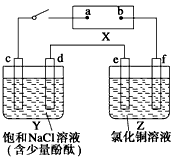
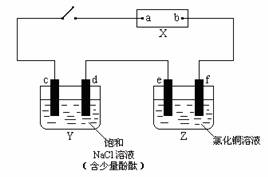
��Ŀ����
��ͼ��X��ֱ����Դ��Y����c��dΪʯī����Z����e��f��������ͬ��ͭ������ͨ��·����d�����Ժ�ɫ��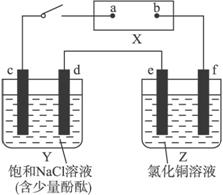
��1���ٵ�Դ��bΪ_____________�����á�������������������������գ���
��Z����eΪ_____________����ͬ�ϣ���
������Y��Z����·�У����������ķ�����d_____________e���á�����������գ���
��2����д��c���Ϸ�Ӧ�ĵ缫��Ӧʽ_________________________________________��
��д��Y�����ܷ�Ӧ��ѧ����ʽ_________________________________________��
��д��Z����e���Ϸ�Ӧ�ĵ缫��Ӧʽ______________________________��
��3���ٵ��2 min ��ȡ��e��f��ϴ������ɡ�������������Ϊ
����Y������Һ���Ϊ500 mL���������Ϊ���䣩�����е�ⷴӦ������v��OH-��=___________________________
��1���ٸ�
����
�ۡ�
��2����2Cl--2e-====Cl2��
��2NaCl+2H2O![]() 2NaOH+H2��+Cl2��
2NaOH+H2��+Cl2��
��Cu-2e-====Cu2+
��3����0.02
��0.02 mol����L��min��-1
�������������Ժ�ɫ����d��Ϊ����ʱӦ�е����ݴ˿�ȷ��dΪ��������һ�����ɽ��1������2�������⡣��3��e��������ӦCu![]() Cu2++2e-�����ᣬf��������ӦCu2++2e-
Cu2++2e-�����ᣬf��������ӦCu2++2e-![]() Cu�����أ�ÿת��2 mol e-���������������
Cu�����أ�ÿת��2 mol e-���������������![]() ����m=
����m=![]() =0.02 mol��Y����2NaCl+2H2O
=0.02 mol��Y����2NaCl+2H2O![]() 2NaOH+Cl2��+H2����2e-,��ת��0.02 mol e-ʱ����OH- 0.02 mol����v��OH-��=
2NaOH+Cl2��+H2����2e-,��ת��0.02 mol e-ʱ����OH- 0.02 mol����v��OH-��=![]() =0.02 mol��L-1��min-1��
=0.02 mol��L-1��min-1��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д� ��2012?����һģ����Na2SO3��Һ�������Ṥҵβ���еĶ����������õĻ��Һ���е��ѭ�������������¹��ս�����ѭ�������������������ӽ���Ĥ���ѭ��������������ͼ��ʾ���������й�˵���в���ȷ���ǣ�������
��2012?����һģ����Na2SO3��Һ�������Ṥҵβ���еĶ����������õĻ��Һ���е��ѭ�������������¹��ս�����ѭ�������������������ӽ���Ĥ���ѭ��������������ͼ��ʾ���������й�˵���в���ȷ���ǣ�������