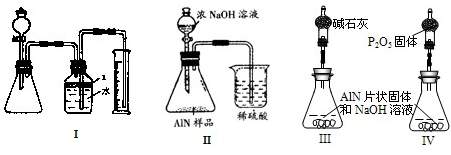
��Ŀ����
��������һ�����͵����ǽ������ϣ����㷺Ӧ���ڼ��ɵ�·���������ڵ����������������뽹̿�ڵ�¯�м����Ƶã�ԭ����Ȳ�����Ӧ����ȫ�����ض�����ɲ�Ʒ�к���̼��Al4C3��Al2O3�����ʣ��ش��������⣺
��1����ӦAl2O3+N2+3C
2AlN+3CO����������
��2��̽������������ķ�Ӧ�����������ɲ����к���NH4+�����������ʵ�鲽�裮
����1��ȡ������Ʒ���Թ��У��μ�ϡH2SO4����Һ������
����2�� ��
��3��AlNҲ������ǿ����Һ��Ӧ��������з���ʽ��
AlN+NaOH+H2O= +
��4��Al 4C3��ˮ��Ӧ���ɼ��飬������CuO��Ӧ�Ļ�ѧ����ʽ���£�
CH4+4CuO
CO2+2H2O+4Cu �ⶨ��Ʒ���йسɷֵĺ����������������£�

�ⶨ��Ʒ�к�������Al4C3�ĺ�������װ�����ӵ�˳��ΪA��C��F��C��D��E����Ҫʵ�鲽�����£�
�ٳ���D����������˳����װ������������������ƿ�У����װ�õ�������
�ڴӷ�Һ©����������ϡ���ᣬֱ�����ٲ�������ʱΪֹ
�۵�ȼF�ƾ���
�ܻ�������һ������N2
���ٴλ�������һ������N2
���ٴγ���D������
���ظ�����ݺ͢IJ�����ֱ��D��������������
�������Ⱥ�˳���Ǣ١� ���ݡ��ޡ��ߣ������ܵ�Ŀ���� ��
��Ϊ�ⶨAlN�ĺ�������ѡ����ʵ�ҩƷ��װ�ã��������������Ӹ�װ�ã���˳��Ϊ��
�� �� ��E������Ϊmg�����C����n g����AlN������������ ��
��1����ӦAl2O3+N2+3C
| ||
��2��̽������������ķ�Ӧ�����������ɲ����к���NH4+�����������ʵ�鲽�裮
����1��ȡ������Ʒ���Թ��У��μ�ϡH2SO4����Һ������
����2��
��3��AlNҲ������ǿ����Һ��Ӧ��������з���ʽ��
AlN+NaOH+H2O=
��4��Al 4C3��ˮ��Ӧ���ɼ��飬������CuO��Ӧ�Ļ�ѧ����ʽ���£�
CH4+4CuO
| ||

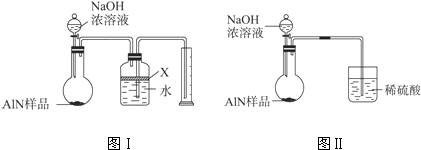
�ⶨ��Ʒ�к�������Al4C3�ĺ�������װ�����ӵ�˳��ΪA��C��F��C��D��E����Ҫʵ�鲽�����£�
�ٳ���D����������˳����װ������������������ƿ�У����װ�õ�������
�ڴӷ�Һ©����������ϡ���ᣬֱ�����ٲ�������ʱΪֹ
�۵�ȼF�ƾ���
�ܻ�������һ������N2
���ٴλ�������һ������N2
���ٴγ���D������
���ظ�����ݺ͢IJ�����ֱ��D��������������
�������Ⱥ�˳���Ǣ١�
��Ϊ�ⶨAlN�ĺ�������ѡ����ʵ�ҩƷ��װ�ã��������������Ӹ�װ�ã���˳��Ϊ��
��������1�����ݻ�ѧ����ʽ��Ԫ�ػ��ϼ۵ı仯������Ԫ�ػ��ϼ۽��͵�Ԫ����������Ϊ��������
��2������笠����ӵļ��鷽�����ʵ�鲽����м��飻
��3��AlN��һ�����õ����ȳ�����ϣ����ܽ���ǿ����Һ����ƫ�����ƺͰ�����
��4��������ʵ��װ������˳��Ͳⶨ�ɷַ����ķ�Ӧ�����жϣ�װ���еĿ�����Ҫ�Ͼ�������Ʒ����ϡ������ȫ��Ӧ�����õ��������ɵļ���ȫ���ϵ�Fװ�÷�Ӧ��������
������ˮ���������������Ͱ����������Ǽ������壬������Ҫ�ü�ʯ�ң����տ�����Ũ���ᣬ�����ؼ��㣮
��2������笠����ӵļ��鷽�����ʵ�鲽����м��飻
��3��AlN��һ�����õ����ȳ�����ϣ����ܽ���ǿ����Һ����ƫ�����ƺͰ�����
��4��������ʵ��װ������˳��Ͳⶨ�ɷַ����ķ�Ӧ�����жϣ�װ���еĿ�����Ҫ�Ͼ�������Ʒ����ϡ������ȫ��Ӧ�����õ��������ɵļ���ȫ���ϵ�Fװ�÷�Ӧ��������
������ˮ���������������Ͱ����������Ǽ������壬������Ҫ�ü�ʯ�ң����տ�����Ũ���ᣬ�����ؼ��㣮
����⣺��1����ӦAl2O3+N2+3C
2AlN+3CO�У���Ԫ�ػ��ϼ۴�0�۱仯Ϊ-3�ۣ����ϼ۽�������������̼Ԫ�ػ��ϼ۴�0�۱仯Ϊ+2������ԭ����
�ʴ�Ϊ��N2 ��
��2���������ɲ����к���NH4+��ȡ������Ʒ���Թ��У��μ�ϡH2SO4����Һ�����ԣ�ȡ��Ӧ�����Һ��������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ������������壬����ֽ������˵������NH4+��
�ʴ�Ϊ��ȡ��Ӧ�����Һ��������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ������������壬����ֽ������˵������NH4+��
��3�����AlN��һ�����õ����ȳ�����ϣ����ܽ���ǿ����Һ����ƫ�����ƺͰ�������Ӧ�Ļ�ѧ����ʽΪ��AlN+NaOH+H2O�TNH3��+NaAlO2��
�ʴ�Ϊ��AlN+NaOH+H2O�TNH3��+NaAlO2��
��4��������װ�����ӵ�˳��Ϊ��A��C��F��C��D��E������D����������˳����װ���������װ�õ������ԣ�������������ƿ�У�װ���еĿ�����Ҫ�Ͼ�������Ʒ����ϡ������ȫ��Ӧ�����õ��������ɵļ���ȫ���ϵ�Fװ�÷�Ӧ��CH4��CuO������Ӧ��CH4+4CuO
CO2+2H2O+4Cu�����ٲⶨ��������Ҫ����IJ���Ϊ���ܻ�������һ������N2���۵�ȼF�ƾ��ƣ��ڴӷ�Һ©����������ϡ���ᣬֱ�����ٲ�������ʱΪֹ�������ܻ�������һ������N2����װ���еĿ����ž�����ֹ��ը��
�ʴ�Ϊ���ܡ��ۡ��ڣ���װ���еĿ����ž�����ֹ��ը��
��Ϊ�ⶨAlN�ĺ���������AlN�ܹ�ˮ���������������Ͱ����������Ǽ������壬�ü�ʯ������ˮ����������Ũ�������հ��������ؼ��㣬����װ������ΪB-D-C��������Ϊmg�����C����ngΪ��������������Ʒ��AlN����������=
��100%=
��100%��
�ʴ�Ϊ��B��D��C��
��100%
| ||
�ʴ�Ϊ��N2 ��
��2���������ɲ����к���NH4+��ȡ������Ʒ���Թ��У��μ�ϡH2SO4����Һ�����ԣ�ȡ��Ӧ�����Һ��������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ������������壬����ֽ������˵������NH4+��
�ʴ�Ϊ��ȡ��Ӧ�����Һ��������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ������������壬����ֽ������˵������NH4+��
��3�����AlN��һ�����õ����ȳ�����ϣ����ܽ���ǿ����Һ����ƫ�����ƺͰ�������Ӧ�Ļ�ѧ����ʽΪ��AlN+NaOH+H2O�TNH3��+NaAlO2��
�ʴ�Ϊ��AlN+NaOH+H2O�TNH3��+NaAlO2��
��4��������װ�����ӵ�˳��Ϊ��A��C��F��C��D��E������D����������˳����װ���������װ�õ������ԣ�������������ƿ�У�װ���еĿ�����Ҫ�Ͼ�������Ʒ����ϡ������ȫ��Ӧ�����õ��������ɵļ���ȫ���ϵ�Fװ�÷�Ӧ��CH4��CuO������Ӧ��CH4+4CuO
| ||
�ʴ�Ϊ���ܡ��ۡ��ڣ���װ���еĿ����ž�����ֹ��ը��
��Ϊ�ⶨAlN�ĺ���������AlN�ܹ�ˮ���������������Ͱ����������Ǽ������壬�ü�ʯ������ˮ����������Ũ�������հ��������ؼ��㣬����װ������ΪB-D-C��������Ϊmg�����C����ngΪ��������������Ʒ��AlN����������=
| ||
| mg |
| 41n |
| 17m |
�ʴ�Ϊ��B��D��C��
| 41n |
| 17m |
���������⿼����������ɺ�̽��������ʵ������жϣ����Ӽ���ʵ�鲽����ƣ�ʵ�鲽������������ǽ���ؼ�����Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ