��Ŀ����
����Ŀ������ʯ(��Ҫ����SrSO4������CaCO3����)�ǻ�ȡ��Ԫ�صĸ��ֻ��������Ҫԭ�ϡ���ش��������⣺
(1)������ɫ��Ӧ���Զ��Լ���ijЩ�����Ρ�����SrSO4ʱ���ȵ���ɫΪ_____(����)�� A�����ɫ B��dz��ɫ C������ɫ D������ɫ
(2)����(SrS)����������Ϳ�ϵ�ԭ�ϣ�SrSO4��̼�Ļ�Ϸ�ĩ�ڸ��������¸��±��տ��������Ⱥ�һ�ֻ�ԭ�����壬 �÷�Ӧ�Ļ�ѧ����ʽ____________________________��
(3)��֪��25��ʱ��K sp(SrSO4)=3.2��10-7��K sp(SrCO3)=1.1��10-10��SrSO4�ķ�ĩ��Na2CO3��Һ��ϼ��ȡ���ֽ�������������ƺ�̼���ȣ���ת�����ʱ�ָ���25�棬���Һ��c(CO![]() )=1.0��10-3mol/L����c(SO
)=1.0��10-3mol/L����c(SO![]() )=______________________��
)=______________________��
(4)������ʯ����Sr(OH)2��xH2O�Ĺ������£�

��֪�� Sr(OH)2 ��Ca(OH)2��ˮ�е��ܽ�����±���
�¶�/(��) | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��(g) | Sr(OH)2 | 0.91 | 1.77 | 3.95 | 8.42 | 20.2 | 91.2 |
Ca(OH)2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 | |
������2Ϊ����CaCO3��SrCO3��д����Ӧ1����SrCO3�Ļ�ѧ����ʽ______________��
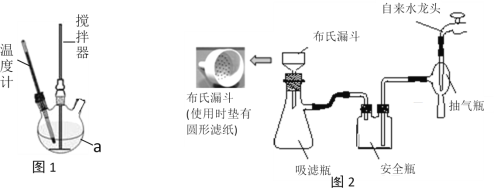
�ڹ���3 ������ˮ������Ϊ�˻�ýϴ�����Sr(OH)2��Һ/span>,��ʱӦ��������ʹ������������,����5����Ҫ�ɷ���______(�ѧʽ),�����ȹ�������Ŀ����_________________________________��
�ۡ�����6����Ҫ�У�________________�����ˡ�ϴ�ӡ����
��ȡm g����Sr(OH)2��x H2O��Ʒ����ˮ���������Na2CO3��Һ����ˡ�ϴ�ӡ�����õ�n g��������x=__________(�ú�m��n��ʽ�ӱ�ʾ)��
���𰸡�A SrSO4+ 4C![]() SrS+4CO�� 2.9mol/L SrSO4 + 2NH4HCO3 = SrCO3��+ H2O + CO2��+ (NH4)2SO4 Ca(OH)2 ��ֹSr(OH)2�ᾧ���������ʧ����߲�Ʒ���� ���½ᾧ
SrS+4CO�� 2.9mol/L SrSO4 + 2NH4HCO3 = SrCO3��+ H2O + CO2��+ (NH4)2SO4 Ca(OH)2 ��ֹSr(OH)2�ᾧ���������ʧ����߲�Ʒ���� ���½ᾧ ![]()
��������
(1)�ȵ���ɫ��ӦΪ���ɫ��
(2)������Ŀ��Ϣд����ѧ����ʽ��
(3)![]() =
= ��������ֵ���м��㣻
��������ֵ���м��㣻
(4)��ҵ�����з�Ӧ1Ϊ��2NH4HCO3ʹSrSO4��Ӧ����SrCO3�����˺�����2Ϊ����CaCO3��SrCO3�����պ����ˮ�����������ø���ʱSr(OH)2��Ca(OH)2�ܽ�ȵIJ�ͬ�����߷��룬�ݴ˽��н��
(1)������ɫ��Ӧ���Զ��Լ���ijЩ�����Σ��ȵ���ɫ��ӦΪ���ɫ��
(2)SrSO4��̼�Ļ�Ϸ�ĩ�ڸ��������¸��±��տ��������Ⱥ�һ�ֻ�ԭ�����壬 �÷�Ӧ�Ļ�ѧ����ʽΪ��SrSO4+ 4C![]() SrS+4CO����
SrS+4CO����
(3)�������֪��![]() =
= �����������c(CO32-)=1.0��10-3mol/L��c(SO42-)=
�����������c(CO32-)=1.0��10-3mol/L��c(SO42-)= =2.9mol/L��
=2.9mol/L��
(4)��ҵ�����з�Ӧ1Ϊ��NH4HCO3ʹSrSO4��Ӧ����SrCO3�����˺�����2Ϊ����CaCO3��SrCO3�����պ����ˮ�����������ø���ʱSr(OH)2��Ca(OH)2�ܽ�ȵIJ�ͬ�����߷��룻
�ٷ�Ӧ1�Ļ�ѧ����ʽΪ��SrSO4+2NH4HCO3=SrCO3��+H2O+CO2��+ (NH4)2SO4��
�ڹ���3������ˮ������Ϊ�˻�ýϴ�����Sr(OH)2��Һ������Sr(OH)2�ܽ�����¶������������������ȹ�������Ŀ���Ƿ�ֹSr(OH)2�ᾧ���������ʧ����߲�Ʒ���ȣ���Ca(OH)2�ܽ�Ⱥ�С�����¶�Ӱ�첻���ԣ�������5����Ҫ�ɷ���Ca(OH)2��
�۲���6Ϊ�˵õ�![]() ���壬��Ӧ���еIJ����ǽ��½ᾧ�����ˡ�ϴ�ӡ����
���壬��Ӧ���еIJ����ǽ��½ᾧ�����ˡ�ϴ�ӡ����
���������֪������ΪSrCO3������Ԫ���غ㣬��![]() �����ʵ�����ͬ���ɵã�
�����ʵ�����ͬ���ɵã�![]() =
=![]() �����x=
�����x=![]() ��
��

����Ŀ���춡���������춡ϩ��ӦΪ��![]()
����ӦΪ�ѽⷴӦ��![]()
![]() ��֪��
��֪��
��ѧ�� | | | | |
���� | 412 | 348 | 612 | 436 |
�Լ����춡�����ⷴӦ��![]() ______
______![]()

![]() ��ͬѹǿ�������춡�����ⷴӦ��ת������ͼ1��ʾ������˵������ȷ����______��
��ͬѹǿ�������춡�����ⷴӦ��ת������ͼ1��ʾ������˵������ȷ����______��
A.����Ӧ����Ӧ���ڵ����������Է�
B.������ѡ���ԵĴ�������Ч���Ƹ���Ӧ�ķ�����������춡ϩ��ѡ����
C.��ͼ1��֪��![]() ��Χ�ڣ��¶Ȳ��䣬ѹǿ�����춡�����ⷴӦ��ת���ʽ���
��Χ�ڣ��¶Ȳ��䣬ѹǿ�����춡�����ⷴӦ��ת���ʽ���
D.ѡ����ʵ��¶ȣ�ʹ�����Ļ����������������춡���ƽ��ת����
![]() ƽ�ⳣ���ı���ʽ��ƽ��Ũ�ȿ�����ƽ��ʱ������ķ�ѹ����
ƽ�ⳣ���ı���ʽ��ƽ��Ũ�ȿ�����ƽ��ʱ������ķ�ѹ����![]() ��ѹ
��ѹ![]() ���ʵ�������
���ʵ�������![]() ��ѹǿ
��ѹǿ![]() ��ͼ1�У�A��״̬�µ��춡�����ⷴӦ��ƽ�ⳣ��
��ͼ1�У�A��״̬�µ��춡�����ⷴӦ��ƽ�ⳣ��![]() ______
______![]() ������λ��Ч����
������λ��Ч����![]() ��
��
![]() ��ѹ��833K�����£��춡���������춡ϩ��ת�������Ŷ����������춡������ı仯�����ͼ2����������Ŷ����������춡����������ӣ��춡��ת���������ӵ�ԭ��______��
��ѹ��833K�����£��춡���������춡ϩ��ת�������Ŷ����������춡������ı仯�����ͼ2����������Ŷ����������춡����������ӣ��춡��ת���������ӵ�ԭ��______��
![]() ����ͼ2�л����¶�Ϊ
����ͼ2�л����¶�Ϊ![]() ��������������ʱ���춡��ת����ͼ��
��������������ʱ���춡��ת����ͼ��
![]() ��ѧ�ҷ��֣���
��ѧ�ҷ��֣���![]() ��
��![]() Ϊԭ�ϣ�����
Ϊԭ�ϣ�����![]() Ϊ����ʣ�����
Ϊ����ʣ�����![]() ����������
����������![]() �ͳ�ѹ�¿�ʵ�ֵ绯ѧ�ϳɰ��������������ı仯����Ϊ���������У��벹���������缫��Ӧʽ��______��
�ͳ�ѹ�¿�ʵ�ֵ绯ѧ�ϳɰ��������������ı仯����Ϊ���������У��벹���������缫��Ӧʽ��______��![]() ��
��
����Ŀ����������������� [ Ag��S2O3��2]3�C���������л����ʣ���AgNO3��Һ��0.1 mol��L�C1 ��pH=6����Na2S2O3��Һ��0.1 mol��L�C1��pH=7�����������ơ�ijС��̽�������Ʒ�����
��ʵ��һ��

��1��AΪAg2S2O3��д������A�����ӷ���ʽ__________��
��2����ʵ������ķ����ó����Թ�a�г�ַ�Ӧ��һ��������__________�������ӷ��ţ��������Ʋ������m����ΪAg2S��Ag��S�����ǵĻ��������Ʋ��������__________��
��3�����Թ�a�����ʳ�ַ�Ӧ���ˣ���������1.1 mL Na2S2O3��Һ�������ã���ɫ�������ܽ⣬��Һ���������ɫ���ǣ��д̼�����ζ�����������ӷ���ʽ���Ͳ�����ɫ���ǵ�ԭ��__________��
���ۣ�Ag2S2O3���ȶ����ֽ����ù��岻����Na2S2O3��Һ��
��ʵ�������֪��Ag2S2O3 ��3S2O32- ![]() 2 [ Ag��S2O3��2]3�C����ɫ��
2 [ Ag��S2O3��2]3�C����ɫ��
ʵ����� | ʵ������ |
i. | ��ɫ�������ɣ���Ѹ���ܽ⣬�õ���ɫ��Һ�� �μ���Լ1 mLʱ��Һ��ʼ�����ػ�ɫ���ж�������� ����1.5 mL����������ɫ������������Ϊ�ػ�ɫ�����ձ�Ϊ��ɫ�� �μ���ϣ����ã��õ���ɫ�������ϲ���ҺpH = 5 |
ii. | ��ɫ�������ɣ�������Ϊ�ػ�ɫ�������õ��ػ�ɫ��Һ���ж�������� |
��4����ƽ���ƶ�ԭ������ʵ��i�м���1.5 mL AgNO3�������ɫ������ԭ��__________��
��5��ʵ��i�У�������0.5 mL AgNO3��Һʱ���õ���ɫ��Һ��������һ��ʱ�䣬�����Ա仯����ϻ�ѧ��Ӧ���ʷ�����������ii��ͬ��ԭ����__________��
��6��������0.1 mol��L�C1 AgNO3��Һ��0.1 mol��L�C1 Na2S2O3��Һ���Ƹñ��ʼ�ʱ���Լ���Ͷ�ϱȺͲ�����__________��