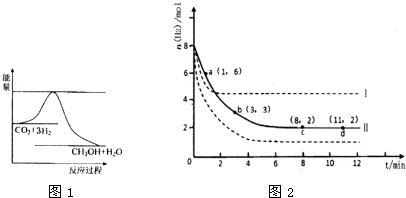
��Ŀ����
Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽���䷴Ӧԭ�����ֽ�������ʵ�飬�����Ϊ2 L���ܱ������У�����2 mol CO2��6 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
 CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
(1)�ӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)��________ mol/(L��s)��
(2)���д�ʩ����ʹCO2��ת�����������(��д��ĸ����)________��
A���������
B�������¶�
C�����ݳ���He(g)��ʹ��ϵѹǿ����
D���ٳ���1 mol CO2��3 mol H2
(3)����˵����ȷ����(��д��ĸ����)________��
A����Ӧ���е�3 sʱ�ﵽƽ��
B��ֻ��ƽ��ʱ��v(CO2)/v(H2)��1��3
C����Ӧ���е�3 sʱŨ�ȹ�ϵΪc(CH3OH)��c(CO2)
D��2 mol CO2��6 mol H2��Ӧ�ﵽƽ��ʱ����73.5 kJ
(2)���д�ʩ����ʹCO2��ת�����������(��д��ĸ����)________��
A���������
B�������¶�
C�����ݳ���He(g)��ʹ��ϵѹǿ����
D���ٳ���1 mol CO2��3 mol H2
(3)����˵����ȷ����(��д��ĸ����)________��
A����Ӧ���е�3 sʱ�ﵽƽ��
B��ֻ��ƽ��ʱ��v(CO2)/v(H2)��1��3
C����Ӧ���е�3 sʱŨ�ȹ�ϵΪc(CH3OH)��c(CO2)
D��2 mol CO2��6 mol H2��Ӧ�ﵽƽ��ʱ����73.5 kJ
(1)0.075
(2)D
(3)CD
(2)D
(3)CD

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��
������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g�� CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
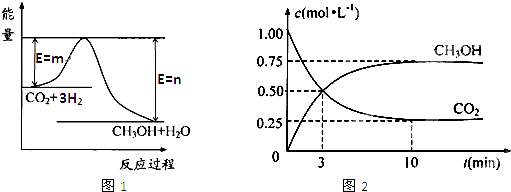
 ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̣�
ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̣�